SLIDES USED IN THIS EXERCISE: 98-13A, 98-16A, 13, 14, VM26, 569, 582, 525, 102, VM29
THERE ARE PRE-RECORDED LECTURES FOR THIS EXERCISE
Please go to the listing page for these to download and view them
Introduction to Muscle Tissue
Smooth Muscle
Striated Muscle
Muscle is another of the four "basic" tissues. "Muscle" is a rather broad term that can be applied to several sub-categories; the categories are based on appearance and structure, but the fundamentals of muscle function are the same for all types.
The two major classifications are smooth muscle (sometimes called "visceral muscle" because of its common occurrence in internal organs) and striated muscle. The striated category is subdivided further into skeletal and cardiac muscle.
Skeletal muscle is sometimes called "voluntary" muscle, because it's subject to conscious nervous system control. It's the principal form in terms of mass as most of the body's bulk is skeletal muscle, used for locomotion and other conscious activities; another word for it is "meat," and skeletal muscle is what you cook on the barbecue on Summer evenings.
Cardiac muscle is a special form of striated muscle, found only in the heart, and will be dealt with in detail in the lab exercise on the circulatory system.
All muscle cells are "excitable cells," i.e., they are capable of responding to appropriate stimulation. The response to this is contraction. The stimulus can vary. It may be an electrical signal or depolarization produced at the surface of the muscle cell by the activity of neuron (which themselves "excitable" cells). Some muscle cells (particularly smooth muscle) can respond not only to neuronal signaling, but to direct chemical or mechanical stimulation. Hormonal signals can cause contraction in some situations.
Muscle is a metabolically dynamic tissue, so as you'd expect it's very well supplied with blood vessels. You'll see many capillaries and small blood vessels in among muscle fibers of all types. In keeping with the functional considerations of muscle as an "effector organ" for the nervous system, you will of course also encounter profiles of nerves and other neural elements. To keep the whole thing structurally intact, there is a good deal of collagenous CT present, as well.
Smooth muscle is found in virtually all organ systems to some extent. It's present in large amounts in some organs of the reproductive tract, especially in the uterus, the walls of the uterine tubes, the wall of the epididymis, and in the ejaculatory apparatus. It forms the bulk of the wall in the gut, and is present to some degree in all blood vessels larger than capillaries. Individual smooth muscle cells are "fusiform" or "spindle shaped" that is, they are longer than they are wide, tapered at each end, and widest in the middle.
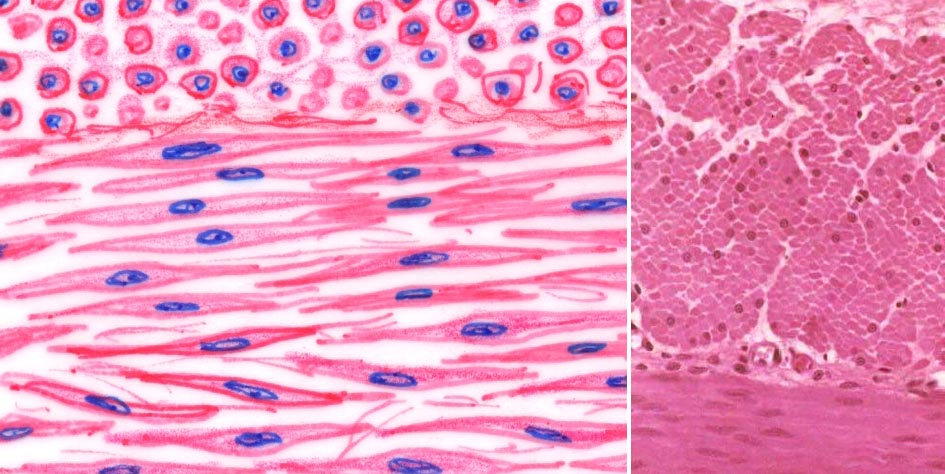
The images at left show smooth muscle in cross section and longitudinal section, diagrammatically and in an actual specimen. The cross sections are at the top of the field in each case. In cross sections, each smooth muscle cell is individually visible as a roughly circular profile, and the size of each profile varies. Some are large, some are small: it depends upon the point in the double-tapered cell that the plane of the section has passed through. Only the largest ones will have nuclei visible in them, because the nucleus occupies the central "fattest" portion of the cell. The size of nuclear profiles is related to the size of the cell profile, as you'd expect.
The boundaries of adjacent cells are not nearly so distinct in the longitudinal section as they are in cross section. In the cross section the separation between fibers is the space occupied by the endomysium around each fiber. It's there in the longitudinal sections, but not easily made out because the cells overlap each other to form a continuous sheet. Furthermore, they're firmly attached to each other for purposes of communication and tissue cohesiveness.

This drawing should make the lateral relationship between two smooth muscle cells a little more understandable. They're represented as they would be seen in a 3-dimensional reconstruction from an electron micrograph. The "fat" part of one lies against the tapered end of the adjacent cell. A gap junction is shown: since these cells have to contract in a coordinated manner and don't have the precise neural control that skeletal muscle cells do, they need to interact with each other: that's the function of the gap junction.
Smooth muscle does have neural input, but it lacks the elaborate neuromuscular junction of skeletal fibers. Neurotransmitters released from the ends of nerve fibers that lie adjacent to the cells (and omitted from this drawing) can be taken into the cells by pinocytosis. Pinocytotic vesicles are a common feature of smooth muscle cells.
Slide 13 shows smooth muscle in longitudinal section on one side and in cross section on the other; examine the cross sections first. Right off you'll notice the varying diameters of the cell profiles, and the fact that only about one in ten shows a nucleus. The reason for the lack of nuclei in a section plane has been discussed. The different sizes result from the tapering shape of the cells; if cut near the tip the profile will be very small, and if near the middle, much larger. If cut in the portion of the cell which contains the nucleus, a nuclear profile will be seen; and since the nucleus is also slightly tapered, the same variability of size results. Now look at the longitudinally sectioned area. The cells lie neatly against each other, with the wide portion of one nested against the narrow parts of its neighbors.
The "wrinkled" look to the nuclei in the longitudinal section is due to contraction of the muscle sheet. This is partly from its natural tonus and partly a result of fixation, i.e., it's an artifact of preparation. Nevertheless, it's a pretty reliable one and it can serve as an identifying feature of smooth muscle in section. Contraction of the cell and compression of the nucleus into a shorter length causes this. In extension, the nuclei are often elongated, and in the relaxed but not contracted state they may have a "cigar shape." Typical smooth muscle cells are about 15-20 microns wide, but they may be up to half a millimeter in length in some cases. This is exceptional; 0.2 mm is more common. These cells have a single nucleus, located at the widest point. Since the nucleus' length is much less than that of the cell itself, a sectioning plane that passes through the cell at right angles to its long axis usually misses the nucleus.
Smooth muscle cells don't show a banding pattern like those of striated muscle (below). They do have internal actin and myosin filaments, but their contractile units are not in register. The relationship of smooth muscle with the surrounding CT is essentially the same as that of skeletal muscle, except that the epimysium doesn't continue into tendons (see below).
You can also see these features in the smooth muscle of the intestinal wall, on slide 40. The outer portions of this section are composed of two layers of smooth muscle oriented at right angles, so that one is cut in cross section, and one longitudinally.
It's very easy to get smooth muscle confused with other tissues, especially regular CT, and one of the easiest traps to fall into is mistaking one for the other. But keep in mind that the nuclei in smooth muscle are inside the cells, and you should have no problems telling it from possible look alikes. It's always best, if you're in doubt, to try to find areas of the tissue cut in cross section; this affords better identification than the longitudinally cut areas.
Now let's look at skeletal muscle, which is the kind Longfellow's blacksmith had in those brawny arms of his. By definition, skeletal muscle is that form which is attached to the skeleton (in common English usage, the word "brawn" has come to mean brute muscle power). Remember that skeletal muscle is only one of the two forms of striated muscle, and "skeletal" and "striated" are not synonymous terms. Cardiac muscle, which is restricted to the heart, is also a striated form.
Before we actually look at skeletal muscle, let's get some terminology straight. The myofiber is the individual skeletal muscle cell, and myofibers are bundled together into groups, or fascicles. These fascicles are in turn "tied" together by CT to form anatomic muscles. The myofiber has internal structure, and each myofiber contains hundreds of myofibrils. (Note that diminutive ending! A "fibril" is smaller than a "fiber"!)
The myofibrils in their turn are composed of smaller structures, the myofilaments. These are the actual contractile parts of the cell, and are principally composed of the two specialized muscle proteins myosin and actin.
Slide 14 is a nice example of skeletal muscle, cut both longitudinally and transversely. At low power, you can make out individual myofibers, or muscle cells. Skeletal myofibers are cylindrical in shape, about 100 microns or so in diameter. The really amazing dimension is their length. Most of them are at least several centimeters long, and it's possible for individual myofibers in large anatomic muscles to be half a meter or more from end to end! In the longitudinally cut areas of slide 14, you should be able to verify the elongated shapes of these cells. The banding pattern of skeletal muscle should be quite obvious. It's a dead giveaway for the nature of this tissue; only the two types of striated muscle show it.
 This field shows the banding pattern characteristic of skeletal muscle very nicely. The alternating dark and light bands so obvious in this image make this tissue hard to mistake for anything else.
This field shows the banding pattern characteristic of skeletal muscle very nicely. The alternating dark and light bands so obvious in this image make this tissue hard to mistake for anything else.
The dark bands are the A and the light ones are the I bands. The I band shows a thin darker Z-line within it; this actually is the demarcation of the limit of the contractile unit of skeletal muscle, the sarcomere.
An A band contains both actin and myosin filaments; an I band contains only actin filaments. The striated appearance comes about because each of these myofibers contains millions of myofibrils whose A and I bands are in register with each other. Each myofibril is composed of millions of sarcomeres in series. A useful mechanical analogy is the arrangements of railroad trains in a switchyard on parallel tracks. If all the trains had the same number of cars, and all the cars were lined up side by side so that they were exactly next to each other, you'd have the same appearance. The cars are the sarcomeres; the trains are the myofibrils, and the switchyard is the myofiber.
The Mechanism of Contraction: The "Sliding Filament Hypothesis"
Previously we discussed the nomenclature divisions of muscle, myofiber, myofibril, and myofilament. There is yet another term to be introduced, the sarcomere. The basic contractile unit of muscle is the sarcomere. In a sense sarcomeres are subunits of myofibrils. They are composed of myofilaments (actin and myosin) arranged in a very specific fashion. Within each myofiber, sarcomeres are joined end to end, thousands of them in innumerable parallel myofibrils. Each one is anchored to the end of the anatomic muscle. Find an area which the muscle is cut longitudinally, and you'll be able to make out the different bands quite well. I bands are the portions of the sarcomeres in which only actin filaments are located, seen as light bands; the darker A bands contain both actin and myosin fibers. The I bands you see here are actually two "half-I bands" back to back between adjacent sarcomeres.
When a striated muscle contracts, the ends of all the sarcomeres approach each other, and the overall sarcomere length is reduced. Since there are thousands of sarcomeres in a myofibril, and all of them shorten, the myofibril shortens quite a bit. Since there are hundreds or thousands of myofibrils, all lined up in register, and they all contract together and all contract the same amount, in the LM you will see the I bands diminish in length, while the A bands do not. This is caused by the "telescoping" of the actin and myosin filaments past each other. The myosin filaments comprising most of the A bands don't move, but the anchor points of the I bands do, and bring the tips of the actin filaments closer together. This mechanical model of contraction is the sliding filament hypothesis.
Anatomic muscles can't expand; they can only contract. That's why they're paired: when one is contracted the other is passively extended to "reset" the sarcomeres to their normal extended length (about 2.2 µm) from the contracted state (about 1.6 to 1.8 µm). In contraction, the ends of the sarcomeres move closer to each other. The I-bands shorten or disappear; the A bands don't change their length. If you have tens of thousands of sarcomeres arrayed end to end, and each of them shortens by a few tenths of a micron, the overall effect on the anatomic muscle is that it shortens by quite a lot. Since the myofibrils are laid side by side, and they all contract simultaneously, the summed force of their contraction is very strong.
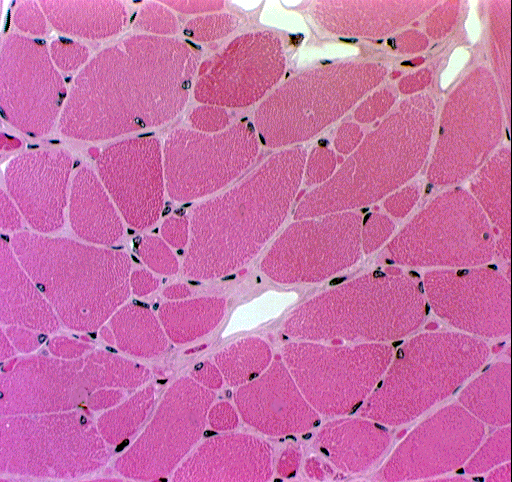
In transverse sections the arrangement of cells in skeletal muscle is even more apparent. Each myofiber is visualizable as a separate unit. The large size of the cells and the positions of the various nuclei present (see below) can be determined easily. If a myofiber nucleus happens to be present in the plane of the section, it's seen inside the sarcolemma, right at the periphery of the cell. The satellite cell nuclei are outside the sarcolemma, but within the basal lamina (which isn't visible without special staining).
Nuclei of Skeletal Muscle
Notice that there are two kinds of nuclei directly associated with the myofiber. The first type is the myofiber nucleus itself, which is clearly located within the fiber. Skeletal muscle cells are multinucleated (in fact, they are syncytial) and may have anywhere from ten to hundreds of nuclei. These are all located right up under the plasma membrane (conventionally called the sarcolemma in all types of muscle cells), because the internal volume of the cell is packed solid with the contractile myofibrils. You can make this out in the two cross sections shown below pretty easily. You'll rarely see more than two or three nuclei in any cross section, because the fiber is so long, that even if there are hundreds of nuclei in it, they're strung out along its length so that the odds of getting a section plane through any given nucleus is small.

The second group of nuclei are those of supporting cells, found between the fibers and in some cases lying in shallow depressions in the surface of the myofiber. Because fibroblasts are so numerous (they make the intercellular collagen network: see below) you'll see them in the CT that surrounds each fiber and bundles of fibers; and of course there will also be nuclei of blood vessel cells, some wandering CT cells, etc., expected in any CT location. Note the large numbers of capillaries between fibers. Skeletal muscle is very well vascularized, because it has such a high oxygen demand, so every myofiber is laid up against a blood vessel somewhere.
There are also reserve cells, incompletely formed myofibers, called satellite cells. These are small cells closely associated with myofibers. They rest in shallow depressions on the surface, and when a need for repair or hypertophy is present, can divide and produce new muscle fibers or add to existing ones.
All muscle depends on CT to transmit the force of contraction. This "intercellular collagen network" is hierarchical in arrangement, and it's diagrammed here. Each myofiber is invested by a delicate CT covering, the endomysium. These collagenous fibers in turn are woven together to demarcate "bundles" of myofibers. The "bundles" are said to be joined together by the perimysium, which is the next higher level of CT. The muscle as a whole is covered with CT, which is of course continuous with the perimysium, and which represents the epimysium. The epimysium is attached to the end points at which the muscle originates or inserts on a bone. Thus the CT provides something for the contracting muscle to "pull on" so that work can be done.
In this scanning electron microscope image (approximately 400x) you can see the surface of a skeletal muscle fiber. The "fuzz" that's obvious on the specimen is the lowest level of the intercellular collagen network (ICN) that surrounds all muscle fibers, and binds the mass into a functional whole. This level, closely associated with and anchored to the surface of the muscle fiber, is the endomysium (from Greek: "endo" = within; "mys" = muscle).
The endomysium of the ICN surrounds every fiber and grades without sharp demarcation into the next level, the perimysium , which in turn is physically linked to the epimysium , the outermost covering. At the ends of an anatomic muscle the epimysium grades into the tendons that anchor it to the bones it moves.
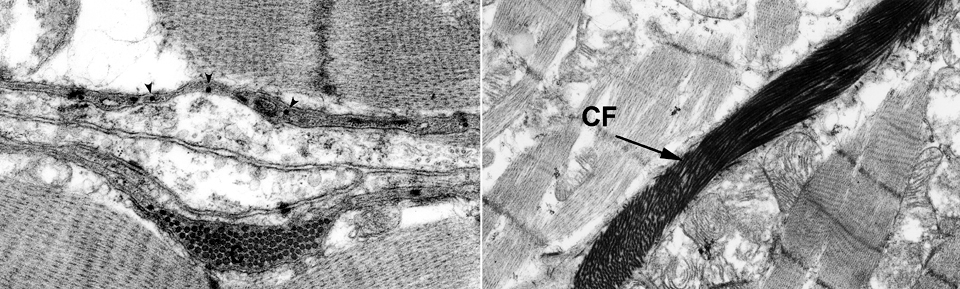
In these transmission electron micrographs (approximately 25,000x and 12,000x) the very intimate relationship of the collagen fibers of the ICN and the surface of the muscle cell is clear. These fibers run individually (small arrows) representing the endomysium; some larger bundles of perimysium (C) are also visible. The collagen is in the space defined on one side by one muscle cell's plasma membrane and on the other side by that of a second muscle cell. The amorphous material surrounding them is essentially the glycocalyx of the cells. Various adhesion molecules and Type IV collagen are involved in keeping these high-strength fibrils in place.
The ICN is crucial to muscle action. If it's not present for some reason, or if the collagen of which it's made is not capable of handling the load imposed on it by muscle contraction, no force can be transmitted through the muscle mass and no work can be accomplished.
Neural Relationships in Skeletal Muscle: Motor End Plates and Neuromuscular Spindles
Skeletal muscle is under nervous system voluntary control. Skeletal muscle must have neural innervation to function. If the axons to a myofiber fail to develop, or are damaged in some way (e.g., by disease or by surgical severing) the muscle cells will wither in the process known as neurogenic atrophy (see below). It's important to understand the structural basis for the interface between a neuron's axon and the myofiber it serves; and the mechanical structures by which the contraction signal is modulated and controlled.
Neuronal processes, like telephone wires, have to end somewhere. In the case of somatic motor fibers, the terminus is on a skeletal muscle cell at the structure called the motor end plate or neuromuscular junction. This is a plaque-like synapse between the end of the axon and the plasma membrane of a muscle cell. The motor end plate represents the final synapse in the motor pathway, between a nerve cell and the myofiber it controls. As with any synapse, transmission of the signal is chemical. In skeletal muscle the neurotransmitter acetylcholine is released into the gap between the terminus of the neuron and the surface of the muscle; this brings about a depolarization of the plasma membrane of the myofiber, internal chemical changes in the cell, and contraction.
 At left is a whole mount preparation of "teased" muscle fiber. (In other words, it's not a sliced section, but individual myofibers pulled apart, placed on a slide, and stained.)
At left is a whole mount preparation of "teased" muscle fiber. (In other words, it's not a sliced section, but individual myofibers pulled apart, placed on a slide, and stained.)
The "bump" on the myofiber in the center of this field is a motor end plate or neuromuscular junction . This is the site of interaction between the nervous system. You can easily make out the axon that comes in to form the motor end plate on this fiber. In vertebrates, each skeletal muscle fiber—no matter how long it may be—has only one such neuromuscular junction.
When acetylcholine is released at this site, the muscle fiber (the post-synaptic cell) undergoes membrane depolarization and a change in ion permeability. The result is a change in electrical charge on the plasma membrane that spreads to cover the entire surface. This in turn results in the release of calcium ions from the myofiber's sarcoplasmic reticulum, triggering the "ratcheting" of actin and myosin filaments past each other, and the overall shortening of that specific myofiber. As with neurons, depolarization and the subsequent contraction it induces is an all or nothing response; when depolarization takes place, all the myofibrils inside that myofiber contract simultaneously and maximally.
The detailed architecture of the neuromuscular junction really requires an electron microscope to see clearly, but some features can be made out in the light microscope. What you see above from slide 542 is a group of motor end plates: they appear as raised plaques on the surface of myofibers. Each end plate has large numbers of nuclei associated with it; some are nuclei of the Schwann cells around the end of the neuron, and others are nuclei of the underlying muscle fiber. An end plate is only associated with one myofiber, and you can see this when you look at them from the top. The oval object at the upper left in this field isn't a motor end plate, it's a muscle spindle. It's part of the control apparatus and will be discussed in more detail below.
The myelin sheath of the axon is lost just before the end plate is formed (you can probably see where this happens). The terminal end of the axon collateral arborizes, and the splayed out ends rest in shallow grooves on the surface of the myofibers. These grooves are actually a complex set of folds in the sarcolemma, the synaptic clefts. When the neuron fires, acetylcholine is released into the space between the plasma membrane of the neuron and that of the muscle fiber; it diffuses across the space, and its release triggers the series of intracytoplasmic events which cause contraction of the sarcomeres.
Take a good look at the image above and the one below at left: notice that the individual axons usually send out
collateral branches. A single axon may therefore innervate several muscle
fibers via its axon collaterals. When the neuron fires, all of these muscle
fibers will contract simultaneously. This aggregation of muscle fibers, all
controlled by one neuron, is a motor unit, and slide 542 shows one. Any given anatomic muscle contains thousands of such motor units, each with
anywhere from 10 to 100 myofibers.  All of the motor end plates in this teased preparation are served by collateral branches of the same axon. Consequently, when that axon fires, all of those myofibers (with one each motor end plate) are also going to fire, simultaneously and maximally.
All of the motor end plates in this teased preparation are served by collateral branches of the same axon. Consequently, when that axon fires, all of those myofibers (with one each motor end plate) are also going to fire, simultaneously and maximally.
The amount of force generated in an anatomic muscle for a given task is dependent on how many motor units in it are "recruited" into use. There may be hundreds or even thousands of motor units in any given anatomic muscle. Decisions on how many to use are made continuously at the CNS level. Not all motor units are "'on line" at any given time; if the task is one that requires little force, only a few may be "recruited" by the brain and as these fatigue, they are shifted smoothly out of service and new motor units switched into use, to maintain the steady level of contraction that muscle has to have. If there's a demand for greater force generation, more and more units can be recruited and the total force the anatomic muscle generates is increased. Eventually, if all the motor units are in use, so that there's no reserve of rested and ready ones, the muscle shows complete fatigue and force generation has to be halted.
Feedback from proprioceptors in among the myofibers and at the joints, tells the brain how much force is being exerted, how much more or less is needed, and when to stop generating force. Coordinating thousands of inputs and outputs is in most species a learned behavior (and it also depends on the degree of development of the cerebellum). Newborn puppies (and human babies) are clumsy because they haven't integrated their nervous system control over skeletal muscle motor units yet. Once the process of final maturation of the CNS is completed, and the "trick" of controlling skeletal muscle precisely is learned, they're able to walk and run.
Muscle Spindles
Anatomic muscles can only contract, not relax; that's why they are paired, so that when one contracts the other is passively extended. By regulating the relative degree of contraction, and the number of motor units involved, any desired level of force can be generated and sustained. Obviously, in order for this system to work, there has to be a great deal of informational input into the central nervous system. The CNS has to know the degree of contraction tension on any given muscle, information that's obtained from a special sensor located deep in the muscle. This sensor is the muscle spindle, a small tapered structure lying in among the myofibers. This information is not only necessary for voluntary functions (such as throwing a baseball or writing a lab handout) but for "automated" ones (i.e., those learned in infancy) such as standing up straight without falling down.
The sense of position and movement is proprioception. Proprioceptive information comes from many sources, of which the skeletal muscle spindle is only one example. One of these can be seen on slide 530 (which also has several motor end plates on it). The muscle spindle on this slide is a wedge shaped collection of nuclei, surrounded by a capsule. A fiber leads from it to join a nearby nerve. The spindle is responsive to mechanical forces stretching its capsule, and sends information on muscular tension to the CNS, which then can adjust the tension generated in the muscle accordingly.
Spindle input is vital to continuous monitoring of body position and movement, and all of this is fed through the "automatic pilot" circuits in the cerebellum. Thus, you need not be consciously concerned about standing up straight, balancing while walking, or not falling off a lab stool. The sensory/motor loops involving the proprioceptors and skeletal muscle make the automatic fine adjustments of muscle tension that are required.
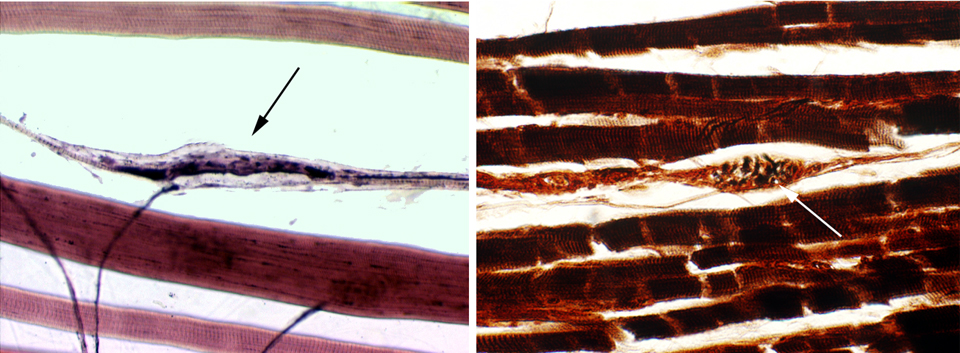
Here's a couple of examples of muscle spindles. The one on the left is from slide 530 (also refer to the image shown earlier for another example). The left image is stained with H&E, the right with a Golgi stain. Both are about 200x in magnification. The muscle spindle is an oval to wedge shaped collection of nuclei, surrounded by a capsule. Nerve fibers to and from it, providing both sensory and motor functionality.
The spindle contains highly modified skeletal muscle fibers. Since it has both sensory and motor supply, it's responsive to mechanical forces that stretch the capsule. Pulling on it sends information on muscular tension to the CNS; the brain can use this input to re-adjust the tension generated in the muscle appropriately for the task. The spindle works like a thermostat: the brain can "set" the spindle to any degree of tension desired, and when the tension varies from the setpoint, use the information the it provides to send contraction signals to motor units.
Spindle input is vital to continuous monitoring of body position and movement. All of this information is fed through the "automatic pilot" circuits in the cerebellum. Normal postural control (standing up straight, balancing while walking, or not falling off a lab stool) is a matter of switching motor units in and out of service to maintain normal muscle tensions. The sensory/motor loops involving the proprioceptors and skeletal muscle make the automatic fine adjustments needed.
As has been mentioned, much of this control learned behavior: in "precocious" animals, such as cattle, the cerebellum is almost completely functional at birth, so that a calf can get up and walk a,most immediately. But in "altricial" species, such as cats and dogs, the newborn is helpless and uncoordinated until the cerebellum completes post-natal development and the circuitry is established.
All muscle is extremely differentiated, and specialized for its function of contraction. As a general rule, very highly specialized cells have little or no capacity to divide, and skeletal muscle is no exception. Once a myofiber has differentiated, that's it: it will never divide again. But the somewhat limited regeneration capacity of skeletal muscle doesn't mean it can't change at all in response to environment or to stresses. It can and does undergo hypertrophy very readily. Hypertrophy, defined as an increase in cell size without a corresponding increase in cell numbers, is the result of the activity of proliferating satellite cells that fuse with exxisting myofibers to increase cytoplasmic volume, and also increased synthesis of contractile units. The highly developed muscles of a bodybuilder or a ballet dancer represent the increased amount of new contractile material within the cytoplasm, but not the production of new myocytes.
Conversely, in atrophy (which can result from disuse or malnutrition, among other causes) the volume of contractile cytoplasm is diminished, but not the number of cells. There are, of course, diseases in which actual cell death occurs, and when this happens, the loss can't be replaced. Loss of muscle cells to necrosis or trauma is made up by the formation of a scar which is mainly collagenous in nature and has no contractile properties.
Since we've been considering the relationships of neurons and muscle fibers, it seems appropriate at this point to look at what happens when the nerve supply is interrupted. This is really pathology, but it illustrates nicely the importance of the neuro-muscular interplay and the structures that control it.

If you've ever broken a leg, and had it in a cast for a few weeks, you've seen how the muscles shrink; this is atrophy of disuse, the counterpart of the hypertrophy of use seen in body builders. Atrophy of disuse is reversible: exercise causes the re-building of lost contractile units. But what's shown here is not reversible. This is permanent neurogenic atrophy, caused by mechanical injury to a nerve during surgery. The muscle cells mo longer get any signals to contract. they start to scavenge the components of their contractile apparatus, and get smaller and smaller, retaining their numerous nuclei. they don't die: they remain metabolically capable of maintaining themselves, in "suspended animation" waiting for the nerve signal that never comes. It's often the result of trauma: depending on the degree of injury, this can happen to a single motor unit or to an entire anatomic muscle.