Objectives for This Exercise
Objectives for This Exercise
 Objectives for This Exercise
Objectives for This Exercise
PRE-RECORDED LECTURE FOR THIS EXERCISE
Endocrine organs are glands whose products are secreted into the blood, to affect tissues distant from the site of secretion. These secretory products, by definition, are hormones.
Because endocrine organs secrete directly into the blood they haven't got the elaborate duct system of exocrine glands: basically they're masses of cells with blood vessels running through them. They're "epithelial organs" under the definition given in Exercise 4, i.e., they're glandular epithelium, not "covering and lining."
It's important to be aware, however, that not every organ that could be considered "endocrine" in its function is necessarily "epithelial" in nature. The whole notion of "endocrine glands" is more than a little wobbly in some respects, because there are organs that we don't think of as "glands," which absolutely do have endocrine functions. These include the kidney and the intestine, among others. The brain is quite rightly considered "endocrine" (for reasons that I hope will be clear shortly) but it's not an epithelial structure.
Two Great Integrating Systems
It can be argued that endocrine physiology is "the ultimate argument against the existence of a rational Supreme Being," on the grounds that no one who was rational would design an animal to work that way. Anyone who's spent time trying to unravel the subtleties or endocrine organs and they way they interact with each other and with other parts of the body will appreciate the kernel of Truth in that wry remark. But some understanding of the structure of the organs of the system gives insight into their relationship to each other, and to the brain, which runs the whole show.
Animals have to respond to a wide variety of stimuli. Some of these occur over short periods (milliseconds to seconds) and others are longer-term (seconds to months). Clearly it is difficult to design a system that has the capacity to adapt to such a broad temporal range: to address these issues, animals have evolved two separate systems with vastly different timing. We normally think of the nervous system as responding rapidly to "short term" phenomena: for example, controlling movement to avoid collisions with objects, and coordinating muscular movement and visual input. The endocrine system is supposedly concerned with seasonal or even yearly adaptations to environmental change.
However, these systems have to operate within the same creature, in an integrated and coordinated way. In reality the brain controls all the responses an animal makes to its environment, including the "long term" ones, and the line between "short" and "long" isn't so clear at that level. There's so much similarity between the way these putatively different body systems are built and work that nowadays the term "neuroendocrine system" or "neuroendocrine axis" has been coined to reflect the situation.
Short term responses are innumerable and easy to illustrate. If you are walking in the woods and trip on a branch, short-term neural signals tell your body which way you're falling, and how to break your fall. If someone throws a ball at you, you use your nervous system to coordinate the movement of your hands to catch it, and to see to it that it doesn't hit you in the face. Long-term responses include such hormonally-regulated activities as coat-color changes, hair molts, and sexual behavior in season breeders.
 But the fact is that all of these responses, regardless of how long they take to come about, require nervous system involvement, in the form of sensory input; and in the end it's the nervous system that triggers the long-term adaptations, using the hormonal controls of the endocrine system as its agent. An excellent example of how long-term adaptation involves both the nervous and endocrine systems participate is the response to cold stress seen in Arctic wolves and sled dogs. Cold—like any other sensation—is perceived directly through nervous system input, using normal pathways of sensory structures and neurons. Short term cold stress is dealt with using behavioral responses (seeking warmth) or physiological ones (shivering). But prolonged input of cold "signal" will cause the brain to produce more of the hypothalamic releasing factor that controls the activity of the thyroid gland. This hormonal response to nervous-system input increases the output of the gland: and an important systemic effect of increased thyroid activity is increased metabolic rate and greater production of body heat. This has obvious advantage to an animal under long term cold stress. Both parts of the "neuroendocrine axis" have to be operating for the dog to adapt to his environment.
But the fact is that all of these responses, regardless of how long they take to come about, require nervous system involvement, in the form of sensory input; and in the end it's the nervous system that triggers the long-term adaptations, using the hormonal controls of the endocrine system as its agent. An excellent example of how long-term adaptation involves both the nervous and endocrine systems participate is the response to cold stress seen in Arctic wolves and sled dogs. Cold—like any other sensation—is perceived directly through nervous system input, using normal pathways of sensory structures and neurons. Short term cold stress is dealt with using behavioral responses (seeking warmth) or physiological ones (shivering). But prolonged input of cold "signal" will cause the brain to produce more of the hypothalamic releasing factor that controls the activity of the thyroid gland. This hormonal response to nervous-system input increases the output of the gland: and an important systemic effect of increased thyroid activity is increased metabolic rate and greater production of body heat. This has obvious advantage to an animal under long term cold stress. Both parts of the "neuroendocrine axis" have to be operating for the dog to adapt to his environment.
In this exercise we'll be concerned with the structure of the pituitary gland, the pineal body, the endocrine portions of the reproductive organs, the endocrine pancreas, the adrenal glands, and the thyroid and parathyroid glands.
We'll start with the pituitary gland, widely referred to as the "Master gland" because its hormonal secretions have as their target organs other endocrine glands. The pituitary controls the hormonal output of many other glands, but it isn't really a true "Master" gland, as we'll see. Nevertheless it's important and its structure is rather elaborate.
The pituitary gland is one of the points of physical and physiological convergence between the nervous and endocrine systems, and its nature and origins reflects this role. The pituitary gland arises from two different embryonic rudiments: one is a depression in the floor of the forming brain and the other is an evagination of the dorsal surface of the forming gut. Consequently, in the finished state, this organ has two parts that are distinctly different in appearance and in nature.
Let's begin the discussion of the structure of the pituitary gland by jettisoning any terminology from previous courses you might have learned, most specifically including the following: "anterior pituitary" and "posterior pituitary." I'm going to be using a more precise descriptive nomenclature, and I'm going to be a stickler about it. The terms we'll use for the different parts of the gland are the adenohypophysis and the neurohypophysis, which more accurately reflect the origins of this organ from, respectively, the gut and the neural tube.
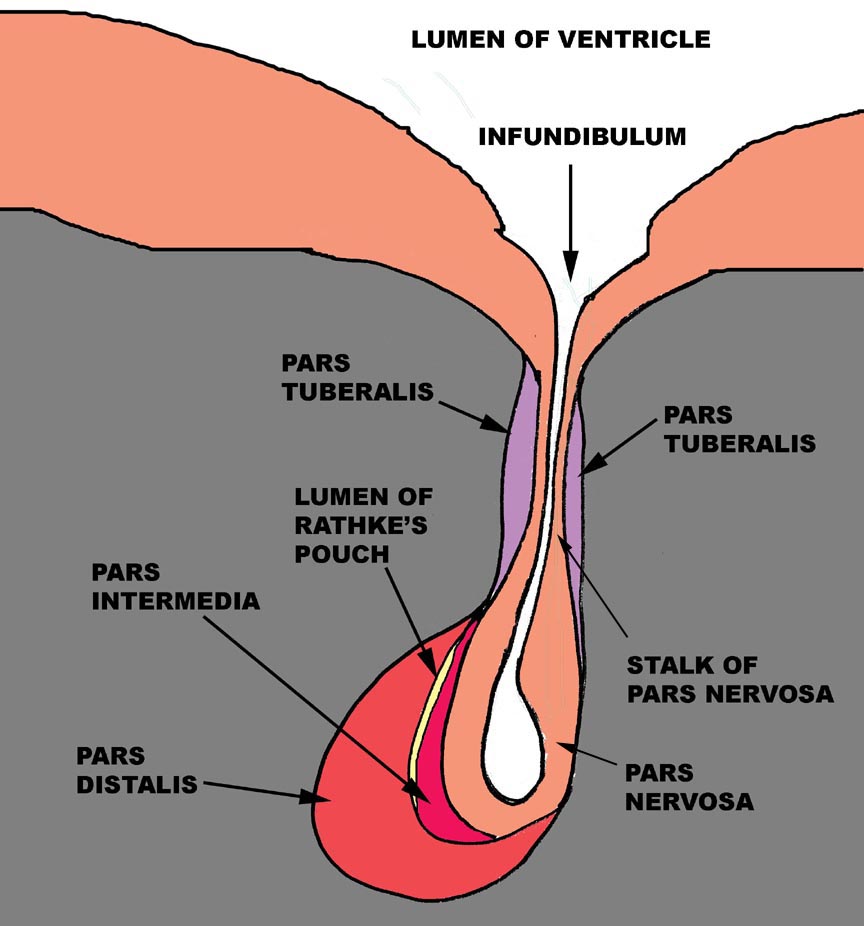 The adenohypohysis is that portion of the gland that comes from the gut:
it is epithelial in nature. The root word "adeno" implies its
origin from epithelium, not neural tissue. The mature adenohypophysis has three distinctly discernible subdivisions: the cranial pars distalis,
the pars intermedia, which is physically associated with the neural
rudiment, and the pars tuberalis, which comes to form a sort of
sleeve-like covering of the stalk from which the neural portion is dependent.
In some species, there may be visible an opening or gap between the pars
distalis and the pars intermedia. This—"Rathke's Pouch"—is the
vestigial remnant of the lumen of the gut pouch. It has no functional
significance and in some species it's obliterated.
The adenohypohysis is that portion of the gland that comes from the gut:
it is epithelial in nature. The root word "adeno" implies its
origin from epithelium, not neural tissue. The mature adenohypophysis has three distinctly discernible subdivisions: the cranial pars distalis,
the pars intermedia, which is physically associated with the neural
rudiment, and the pars tuberalis, which comes to form a sort of
sleeve-like covering of the stalk from which the neural portion is dependent.
In some species, there may be visible an opening or gap between the pars
distalis and the pars intermedia. This—"Rathke's Pouch"—is the
vestigial remnant of the lumen of the gut pouch. It has no functional
significance and in some species it's obliterated.
The neurohypophysis is that part of the organ derived from the brain, and unlike the adenohypophysis, it retains its connection to the tissue of origin, via a long stalk. The neurohypophysis has a lumen that's continuous with the lumen of the brain's third ventricle. The lumen of the neurohypophysis, like the ventricles of the brain, is lined by ependymal cells. This part of the pituitary gland, in an anatomic sense, may be considered a part of the brain, and its histologic appearance confirms this. It consists mainly of nerve fibers with associated glial elements. It is a dependent part of the median eminence of the hypothalamus.
The cells of each pituitary region have distinct hormonal products associated with them. These are summarized elsewhere.
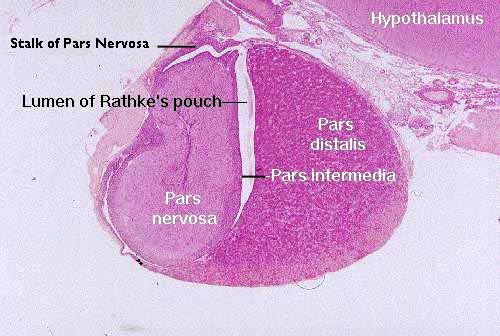 A representative section of the pituitary gland is
seen in slide 155, the pituitary gland from a cat. The cat is one of the species in which the residual lumen of Rathke's pouch (Martin H. Rathke, 1793-1860, a German anatomist and pathologist) remains open. The space is obliterated in other species, or sometimes reduced to a small series of fluid filled cysts.
A representative section of the pituitary gland is
seen in slide 155, the pituitary gland from a cat. The cat is one of the species in which the residual lumen of Rathke's pouch (Martin H. Rathke, 1793-1860, a German anatomist and pathologist) remains open. The space is obliterated in other species, or sometimes reduced to a small series of fluid filled cysts.
This section is taken vertically through the gland, and the section on your slide may or may not have come from the approximate midline of the organ. Begin by holding the slide up to the light to determine this general outline of the organ; next scan it at low magnification. You can compare what you see on this slide with the diagram above. The cranial portion, consisting mostly of the pars distalis, is to the right, and the hypothalamus of the brain is at the top of the image.
 In life the pituitary gland dangles below the brain, comfortably lodged in a pocket of the sphenoid bone and surrounded by a CT capsule which serves as the route for blood vessels. As nice as the section above is, unfortunately the stalk that connects the pars nervosa to the hypothalamus isn't well demonstrated. So here's another example, in which the stalk is more easily seen.
In life the pituitary gland dangles below the brain, comfortably lodged in a pocket of the sphenoid bone and surrounded by a CT capsule which serves as the route for blood vessels. As nice as the section above is, unfortunately the stalk that connects the pars nervosa to the hypothalamus isn't well demonstrated. So here's another example, in which the stalk is more easily seen.
 The stalk is hollow, and at the top it communicates with the third ventricle of the brain. At the bottom it ends blindly inside the pars nervosa. It's lined with ependymal cells and filled with cerebrospinal fluid. If you were standing inside the brain, you could look "down" into the hole in the floor of the third ventricle, and if you were to shine a flashlight down that hole, you could see the bottom.
The stalk is hollow, and at the top it communicates with the third ventricle of the brain. At the bottom it ends blindly inside the pars nervosa. It's lined with ependymal cells and filled with cerebrospinal fluid. If you were standing inside the brain, you could look "down" into the hole in the floor of the third ventricle, and if you were to shine a flashlight down that hole, you could see the bottom.
In this image you can see part of the pars intermedia, and the upper end of the pars distalis as well. The pars tuberalis surrounds the stalk in most species, sort of like a sleeve. The degree of envelopment of the stalk varies from one species to the next.
Pars Distalis
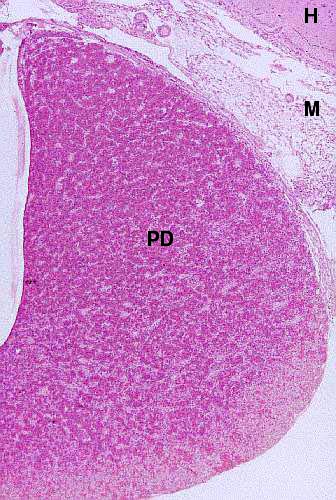 The most prominent area, consisting of a uniform mass of relatively basophilic
cells, is the pars distalis. There may be a space or cleft next to it
(this is the residual lumen of the gut rudiment) and on the opposite side of
the cleft you should be able to see the narrow region of the pars intermedia.
The most prominent area, consisting of a uniform mass of relatively basophilic
cells, is the pars distalis. There may be a space or cleft next to it
(this is the residual lumen of the gut rudiment) and on the opposite side of
the cleft you should be able to see the narrow region of the pars intermedia.
Here's the pars distalis (PD), at low magnification. At this level of magnification you see it as a more or less homogeneous mass of cells (which it most decidedly isn't). At somewhat higher magnifications it will be possible to make out the presence of blood channels among the masses of cells (see below). A bit of the hypothalamus (H) is visible; and so is some of the meningeal covering of the brain (M).
Since the pituitary hasn't got any "plumbing" in the form of excurrent ductwork, blood is used to transport its products. It has a cord-and-sinus architecture is designed to maximize contact between the blood and the cells of its parenchyma. It uses a "cord and sinus" arrangement of blood spaces (sinuses) between cords of functional cells.
 In this image you can see the cord and sinus arrangement of the pars distalis pretty well. The blood sinuses are a form of capillary, and blood moving though them is broken up into many small channels. The channels are lined with fenestrated capillary endothelium.
In this image you can see the cord and sinus arrangement of the pars distalis pretty well. The blood sinuses are a form of capillary, and blood moving though them is broken up into many small channels. The channels are lined with fenestrated capillary endothelium.
This arrangement assures that anything in the blood flowing through has pretty much unimpeded access to the cells in the cords, and vice versa: the cells in the cords have the opportunity to put their products (pituitary hormones) into the blood instantly. Unlike the case of the liver sinuses, the endothelium is continuous; there aren't any breaks where the cells in the cords are directly bathed in blood. Nevertheless, for all practical purposes the barrier to movement of material in either direction is minimal. This same cord-and-sinus architecture is found in the rest of the adenohypophysis, i.e., the pars intermedia and the pars tuberalis.
The cells in the cords are of several types. An old-fashioned nomenclature based on the staining reaction of these cells is still useful in the context of histology, but using immunolabeling methods it's possible to associate specific cell types with specific hormones in a way that no H&E preparation can.
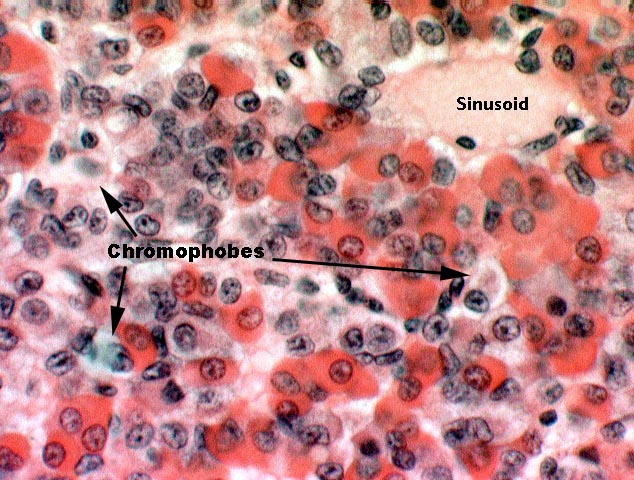 Using the older terms, chromophils are those which have taken up stain; they can be further subdivided into acidophils and basophils, which stain pink or purple respectively.
Using the older terms, chromophils are those which have taken up stain; they can be further subdivided into acidophils and basophils, which stain pink or purple respectively.
In the image at right the acidophils are brightly stained with the eosin dye, and the basophils a more subdued color, resulting from their taking up hematoxylin. The sparse chromophobes are the unstained cells in here. Each type produces different hormones that have different target organs in the periphery, a list of which has been given elsewhere. In the pars distalis, specifically, the acidophils produce growth hormone and prolactin; the basophils produce thyroid stimulating hormone, adrenocorticotropic hormone, and gonadotropic hormones.
Adenophypophyseal Response To Hypothalamic Releasing Factors
The cells of the pars distalis, pars intermedia, and pars tuberalis (i.e., the entire adenohypophysis) are themselves the "target cells" for hormones: but the hormones to which they respond are produced in the brain itself. The adenohypophysis doesn't receive its input of blood from arteries, but instead from an arrangement of two capillary beds in succession.
In the median eminence of the hypothalamus, i.e., up in the actual brain, arteries end in capillary beds. The venous drainage from these capillaries in turn is sent through the sinusoids of the adenohypophysis, including most especially those in the pars distalis. The first capillary bed drains into the second, creating a portal system. Its functional significance for the pituitary gland lies in the fact that the hypothalamus itself produces hormones. Those hormones—the hypothalamic releasing factors—target the cells of the pituitary and no others.
The portal arrangement of blood vessels means that anything put into the blood in the hypothalamus will immediately be carried to the adenohypophysis, and have access to the cells lining the sinusoids. That's exactly what happens; the hypothalamus produces hormones, and the target organs of these hypothalamic releasing factors are specifically the cells of the adenohypohysis. Dumped into the blood, and carried via the portal system to the sinusoids, they are chemical signals which in turn cause the cells of the pars distalis and pars intermedia to elaborate and release their hormones into the sinusoids. The brain therefore directly controls output of the pituitary gland. Put another way, the brain is an endocrine organ in its own right.
Pars Intermedia and Pars Tuberalis
The pars intermedia, pressed up close to the neurohypophysis, is another epithelial area. Its staining reaction is distinctly different from the pars distalis because there are normally no acidophilic cells visible. The pars intermedia grades into the pars tuberalis without a definite boundary.
 This image shows the pars intermedia, that part of the adenohypohysis that's pressed closely up against the pars nervosa. You see it here as a thin strip of somewhat basophilic cells.
This image shows the pars intermedia, that part of the adenohypohysis that's pressed closely up against the pars nervosa. You see it here as a thin strip of somewhat basophilic cells.
The much-larger pars distalis is to the right in this field and the residual lumen of Rathke's pouch is visible. In humans the lumen may persist as a few small cysts along the border between the pars intermedia and the pars distalis. It has no functional significance, whether it's present or not.
The pars distalis, pars intermedia and the pars tuberalis are all derived from a rudiment arising from the cranial end of the gut, as a dorsal evagination of the stomodeum. Thus the adenohypophysis arises from embryonic ectoderm. The ventral downgrowth of the neural tube that forms the neurohypophysis is also ultimately of ectodermal origin.
The pars intermedia secretes MSH, or "intermedin," which has some similarities to ACTH in structure and in activity. The pars intermedia is more extensive in the domestic animals than in humans. The pars tuberalis is not yet known to secrete any hormones. Even though it may be similar to the pars intermedia, the pars tuberalis is quite clearly distinct from the adjacent pars nervosa; it forms a "cuff" or "sleeve" around the stalk of the pars nervosa itself.
 We've been looking at subdivisions of the adenohypophysis, and it's time to examine the neurohypophysis, principally the pars nervosa. The pars
nervosa arises as a ventral outpouching of the floor of the brain, and it
retains its connection with the brain. The lining cells of the pars nervosa are
the same sort of ependymal cells found lining the central nervous system. If
you have a favorable section, you may be able to trace the connection between
the space in the pars nervosa and the brain's third ventricle. In addition to having a lining of ependymal cells, it's filled with cerebrospinal fluid. So, for all intents and purposes, the pars nervosa remains part of the brain physically.
We've been looking at subdivisions of the adenohypophysis, and it's time to examine the neurohypophysis, principally the pars nervosa. The pars
nervosa arises as a ventral outpouching of the floor of the brain, and it
retains its connection with the brain. The lining cells of the pars nervosa are
the same sort of ependymal cells found lining the central nervous system. If
you have a favorable section, you may be able to trace the connection between
the space in the pars nervosa and the brain's third ventricle. In addition to having a lining of ependymal cells, it's filled with cerebrospinal fluid. So, for all intents and purposes, the pars nervosa remains part of the brain physically.
The pars nervosa is neural tissue. It consists of well vascularized fiber tracts. Most of these are axons coming from neurons whose cell bodies are located in the hypothalamus. These axons carry materials made in the brain down into the deep part of the pars nervosa. Unlike most other axons, they don't form synapses with other neurons, however.
When these axons reach the pars nervosa they end in contact with blood capillaries. Now, this is a little unusual: it implies direct release of neuron products into the blood, and that's exactly what happens in the pars nervosa.
The pars nervosa is a place of storage and release of hormones made in the brain. The two hormones associated with it are oxytocin and vasopressin, small cyclic peptides that affect smooth muscle and the epithelium of the kidney tubules respectively. These hormones are made in neurons of the supraoptic and paraventricular nuclei in exactly the same way neurotransmitters are made, and then carried to the pars nervosa in the axons that end at the surface of capillaries. Upon appropriate stimulation, they release their products directly into the blood at these sites. Thus the brain is acting as an endocrine organ, and releasing its products at a dependent site. That is, not only does the brain control the pituitary gland: it directly controls other targets by releasing specific blood-borne hormones. Control of release is bade on input via normal nervous system channels. Appropriate signals coming in from specific receptors in the periphery trigger release of the hormones, completely bypassing the portal circulation. The brain is an independent endocrine organ capable of acting without the mediation of the pituitary. The pituitary is, at best, a sort of "Overseer Gland" whose job it is to transmit the orders of the true "Master Gland," the brain.
After that rather exhausting tour of the pituitary, it's something of a relief to examine a much less complicated endocrine organ, the pineal gland, sometimes called the epiphysis cerebri. You'll see it on slide 618. Grossly, the pineal gland is a sort of cone shaped structure in the midline of the brain, projecting from the roof of the diencephalon. The name comes from the Latin pinus, a pine tree, from its vague resemblance to one of these.
At a magnification low enough to see the entire organ, it's not much to look at: a nondescript lump of uniformly stained tissue. There's a capsule of sorts and some internal stroma, and that's about it.
 Histologically speaking, the pineal gland is uninteresting; about 95% of the
cells are the pinealocytes, the functional cells which manufacture the
pineal hormone melatonin (5-methoxy-N-acetyl-tryptamine).
Histologically speaking, the pineal gland is uninteresting; about 95% of the
cells are the pinealocytes, the functional cells which manufacture the
pineal hormone melatonin (5-methoxy-N-acetyl-tryptamine).
These cells are large and lightly stained in H&E, with a rounded nucleus. The remaining cells of the organ (about 5%) are astrocytes, which cannot be seen on this H&E stained slide.
At left is a somewhat more interesting image, and you see in it what look like areas of calcification. They look like that because that's exactly what they are.
The most obvious aspect of the histology of the pineal gland is the presence of the acervuli cerebri (from Latin, "acervus," a heap). This is more prosaically (and equally correctly) called "brain sand." It's aggregations of calcareous secretions that are easily seen with the microscope. These are not a degenerative change; although calcification is in fact pathological in other tissues, in this case it isn't.

Brain sand is present in almost all adult mammals. The mineral composition makes these aggregations radio-opaque, giving them some clinical significance as a radiographic"marker" for the midline of the brain. Displacement is easily seen on radiographs, and is a possible indication of the growth of a tumor.
The pineal gland is physically part of the brain and it receives input from nerve fibers that "inform" it of the level of light outside. In what we featherless bipeds like to call "primitive" animals it's often directly light sensitive, placed in a portion of the skull where the overlying bone is thinned out and translucent, so that light rays penetrate to reach the pineal. But in mammals this isn't the case and input is via nerve fibers from the visual coordinating center. However it receives input, the pineal body's responsiveness to light is important in its role as the coordinator of the circadian and seasonal rhythms of the body. It "resets" the internal clock on a daily basis by its cyclic response to light.
Function Of The Pineal
For many years exactly what the pineal gland does in mammals was a complete mystery, and the story behind the reason why this was the case is an interesting illustration of how really obvious things get overlooked. It happened because the standard animal for most physiologic investigations is the white rat, and millions of them are used every year.
 Decades of experimentation with rats failed to reveal any function at all for the pineal gland: none of the thousands of experiments that were done seemed to make any sense at all and no data was generated that was physiologically meaningful. For many years the conclusion was that in mammals the pineal gland was "vestigial" and "nonfunctional." You will still occasionally hear this statement made, and even see it in books!
Decades of experimentation with rats failed to reveal any function at all for the pineal gland: none of the thousands of experiments that were done seemed to make any sense at all and no data was generated that was physiologically meaningful. For many years the conclusion was that in mammals the pineal gland was "vestigial" and "nonfunctional." You will still occasionally hear this statement made, and even see it in books!
But.......all the investigators who used rats for pineal gland studies missed one crucial point: when the scientific community demands millions and millions of rats per year, someone has to go into the rat business to supply them. Now, naturally, with that demand there's a lot of money to be made in the breeding of white rats. The people who run the rat farms want rats who are truly dedicated to their jobs, rats who will be tireless in their efforts to make new rats, and therefore to raise the company's profit margins. Any rat whose libido is so low that he or she is incapable of, or unwilling to, spend every single waking moment engaged in—you should pardon the expression—reproductive behavior is deadwood, and represents a monetary loss to the rat factory. Such slackers get weeded out of the population because they aren't profitable to maintain.
What therefore happened, albeit inadvertently, was that over tens of thousands of generations white  rats were being bred at least as much for their enthusiasm for reproduction as for anything else. In these dedicated and self-sacrificing rats, the gonadal suppressive activity of the pineal gland was being selectively bred out of the line, and they were functionally "pinealectomized." Therefore any tests of "what the pineal does in mammals" using the white rat model were meaningless.
rats were being bred at least as much for their enthusiasm for reproduction as for anything else. In these dedicated and self-sacrificing rats, the gonadal suppressive activity of the pineal gland was being selectively bred out of the line, and they were functionally "pinealectomized." Therefore any tests of "what the pineal does in mammals" using the white rat model were meaningless.
Light finally began to dawn on investigators (sorry, I couldn't resist) when they decided to try it with Syrian hamsters (Mesocricetus auratus) instead of rats. These little rodents are much less inbred than any commercial rat strain, and are even more dedicated to—ahem—non-stop reproductive behavior than rats. Lo and behold, suddenly the nature of the pineal gland's products, and the relationship of gonadal suppression to light and dark cycles became clear.
This is an object lesson in how an experimental design that's not thought completely through can give reliable, repeatable, and precise data, from which consistent and thoroughly incorrect conclusions can be drawn.
The pineal gland arises as a dorsal outgrowth of the caudal portion of the diencephalon. In the adult animal it lies in the midline of the roof of the third ventricle. Evolutionarily, it's a very old and highly conserved organ, and it's found in some form in all of the vertebrate classes. And it presents another excellent example of the integration of the nervous and endocrine systems.
In amphibia and in many reptiles, the pineal body is directly light sensitive; it's sometimes been called the "third eye" or "parietal eye." In these animals (which literally have "an eye in the back of their head") the photosensitive pineal gland, lying just beneath the integument of the head, resets the biological clock via nervous impulses to higher centers. It thus controls circadian rhythms.
In mammals this direct photosensitivity has been lost, but the pineal gland is still functionally related to light cycles. The pineal gland in mammals receives neural signals originating in the visual nucleus of the brain, and mediated by the sympathetic side of the autonomic nervous system. Thus the pineal gland "knows" whether it's light or dark, and what the proportion of day to night is. The English romantic poet Tennyson observed that "In Spring a young man's fancy lightly turns to thoughts of love," and considering the response of the pineal body to light, and its influence over gonadal activity, this should not be too surprising. The effect is most pronounced in seasonal breeders.
The pineal gland appears to exert hormonal regulation of the development of the gonads, by suppressing the activity of cells which produce gonadotropin releasing hormone in the hypothalamus. If no GNRH is released, no gonadotropins are released, and hence the gonads don't reach a functional state. Melatonin is therefore a gonadosuppressive hormone. Pineal abnormalities causing decreased secretion of melatonin will cause early onset of puberty, and administration of exogenous melatonin delays it.
The altered day length of the Spring triggers increased sexual activity in many animals. Production of the enzyme which catalyzes the synthesis of melatonin increases in the dark, so the short days and long nights of Fall and Winter mean increased melatonin production. Conversely, the lengthening days of Spring and Summer mean less darkness and hence less melatonin. Decreased melatonin synthesis means more GNRH, more production of gonadal hormones, and more vigorous sexual behavior in species which breed seasonally in the Spring. In such animals, the suppressive effect of melatonin is less pronounced in the late Winter due to poorly understood physiologic changes.
Endocrine Tissues of the Reproductive System
Both the testis and the ovary are important endocrine organs, and the hormones they produce are important in normal sexual activity, in maturation of the adult body form, and in controlling estrus and pregnancy.
The testis is seen on slide 51. The bulk of this slide consists of seminiferous tubules, easily identified by their circular profile and characteristic cell types. (These will not be dealt with here, since you will cover them in detail in the reproductive system lab exercise.)
Interstitial cells produce the "guy hormone" testosterone. Testosterone has effects on a number of organs, including hair follicles, the reproductive organs, and the musculoskeletal system. A surge in testosterone production at the time of puberty is the trigger for development of secondary sex characteristics of males, such as beards and manes, as well as changes in behavior.
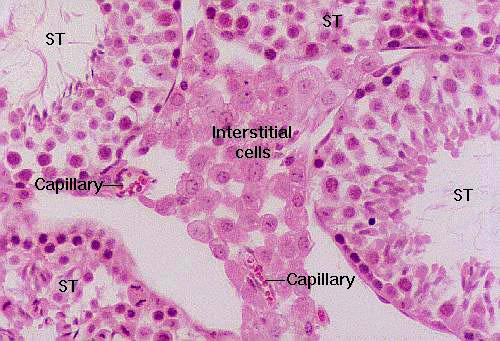 Here's a cluster of interstitial cells wedged into the space between three seminiferous tubules (ST). Testosterone is a steroid; since these are steroid-secreting cells, they don't show the basophilic reaction you would expect from protein making ones. In the electron microscope, they show large amounts of smooth endoplasmic reticulum, the subcellular organelle involved in steroid synthesis. Notice the presence of a couple of capillaries in here, both for nutrient and waste exchange, and also to carry the glandular product to the target organs.
Here's a cluster of interstitial cells wedged into the space between three seminiferous tubules (ST). Testosterone is a steroid; since these are steroid-secreting cells, they don't show the basophilic reaction you would expect from protein making ones. In the electron microscope, they show large amounts of smooth endoplasmic reticulum, the subcellular organelle involved in steroid synthesis. Notice the presence of a couple of capillaries in here, both for nutrient and waste exchange, and also to carry the glandular product to the target organs.
The interstitial areas are well vascularized, and between the clusters you
should be able to make out  capillaries containing erythrocytes. Near the
capillaries you'll find small clusters of cells. These are the interstitial
cells, the source of the male steroid hormone testosterone.
Ultrastructural examination will show that the interstitial cells have the
characteristic features associated with steroid synthesis, especially the
presence of large quantities of smooth endoplasmic reticulum.
capillaries containing erythrocytes. Near the
capillaries you'll find small clusters of cells. These are the interstitial
cells, the source of the male steroid hormone testosterone.
Ultrastructural examination will show that the interstitial cells have the
characteristic features associated with steroid synthesis, especially the
presence of large quantities of smooth endoplasmic reticulum.
Ovary and Corpus Luteum
The ovary is a major endocrine organ, and the variation of hormones in the female reproductive cycle is of great importance, complex enough to require its own exercise We'll deal with the ovarian secretory tissues in the exercise devoted to the female reproductive tract. Endocrine Pancreas
Endocrine PancreasIn the exercise on digestive system glands we looked at the pancreas in light of its exocrine function, the source of digestive enzymes. But this organ is also an endocrine gland. Examine slide 34; while the bulk of the pancreatic tissue is exocrine in nature, the endocrine pancreas is distributed through the mass of the organ as "islands" of lighter staining material. These pancreatic islets are easily seen at low power. At higher magnification several characteristics of the islets can be seen.
Islets are more or less set off from the surrounding exocrine tissue by a thin "capsule" of CT. They're extensively vascularized, much more so than the surrounding exocrine tissue: an islet is essentially a capillary bed surrounded by secretory cells.
The cells of the islets are not easily distinguished from one another in H&E preparations, but immunostaining using the peroxidase reaction (below) to localize their products can tell them apart. Four types are known to exist. The principal ones are the A and B cells; the A cells make glucagon (below right) and the B cells make insulin. These two antagonistic hormones are vital to normal carbohydrate metabolism.

Slide 1202 is a section of adrenal gland. The adrenal gland has a distinct cortex and medulla, each of different embryologic origin and different function. Again, in the adrenal, as in the pituitary, we can see the physical and physiological convergence of the two integrating systems.

Even at low magnification, there's an obvious distinction between the adrenal cortex and its medulla. They arise from different embryonic rudiments, have vastly different functions, and respond to different signals, so it's not too surprising that this should be the case. In this field you see about one third of the total organ. The cortex, as the name implies (it means "rind" in Greek)  surrounds the medulla. The adrenal glands are embedded in the fat at the cranial end of the kidney, hence are sometimes referred to as "supra-renal glands." Each has its own capsule (visible here as wisps of CT at the edges) and blood supply. The medulla and the cortex have separate arterial supplies, but share a common venous drainage.
surrounds the medulla. The adrenal glands are embedded in the fat at the cranial end of the kidney, hence are sometimes referred to as "supra-renal glands." Each has its own capsule (visible here as wisps of CT at the edges) and blood supply. The medulla and the cortex have separate arterial supplies, but share a common venous drainage.
As you examine this slide, note the extensive vascularization of both parts of this organ. As in any endocrine organ, release of hormones to the blood is rapid, and the great degree of vascularity permits all cells to have good access to the circulation. The cells of the cortex and the medulla have the familiar cord and sinus arrangement, which facilitates contact between the cells that make hormones and the blood flowing past them. The cells of the adrenal cortex all synthesize steroid hormones, and have a "foamy" appearance in some sections. This is due to the extensive lipid material in them, and to the numerous SER and Golgi profiles in their cytoplasm.
The adrenal cortex and the medulla have separate and distinct arterial supplies, related to the different origins and functions of the two parts, discussed below. You may be able to see some of the medullary arteries which run straight through the cortex, bypassing it completely, and ramify into the sinusoids of the medulla. The cortex is supplied by cortical arteries which enter the organ via the capsule and discharge their blood into the sinusoids of the zona glomerulosa (see below). Venous outflow from the cortex is mixed with that of the medulla, and carries secretions from both parts.
The Adrenal Cortex: Capsule and Zona Glomerulosa
The adrenal gland as a whole is surrounded by a distinct CT capsule, which sends a few septa into the parenchyma of this gland; generally, though, there is little internal lobulation. The cortex is quite distinct from the medulla and it's divided into three zones: the zona glomerulosa, zona fasciculata, and zona reticularis.
The layers of cells immediately beneath the capsule are organized into the zona glomerulosa, or "region of little globes." They form arcades or small arches of cells.
This slide is stained with a connective tissue routine that highlights the capsule and the stroma of the adrenal cortex. The zona glomerulosa cells are also easily distinguished from the deeper zona fasciculata.
Zona Fasciculata and Zona Reticularis
Deep to this layer is a second distinct region where the cells form somewhat more regular rows, radiating away from the center of the gland. This is the zona fasciculata, the name coming from the Latin word "fascis," a bundle of sticks. Like sticks bound together, these cords of cells run in roughly the same direction.
The cells in the zona reticularis, that region of the cortex closest to the medulla, lose this regular arrangement, and are organized into anastomosing cords to form a network-like zona reticularis. Separating the rows and cords of cells are irregular vascular channels, or sinusoids similar to those in the liver.
 The adrenal medulla comes from an entirely different embryonic rudiment than
does the cortex. The cortex arises from a condensation of embryonic mesoderm,
but the medulla, ultimately, is of neuroectodermal origin. It develops from cells of the neural crest that migrate into the forming adrenal gland. As a result it has
different properties than the cortex and its secretions are triggered by very different stimuli. In the
fully formed organ, innervation of the medulla by the autonomic nervous system
is direct; there is no intervening ANS ganglion as in other target organs. In reality, the adrenal medulla can be though of as a sort of ganglion combined with its effector organ.
The adrenal medulla comes from an entirely different embryonic rudiment than
does the cortex. The cortex arises from a condensation of embryonic mesoderm,
but the medulla, ultimately, is of neuroectodermal origin. It develops from cells of the neural crest that migrate into the forming adrenal gland. As a result it has
different properties than the cortex and its secretions are triggered by very different stimuli. In the
fully formed organ, innervation of the medulla by the autonomic nervous system
is direct; there is no intervening ANS ganglion as in other target organs. In reality, the adrenal medulla can be though of as a sort of ganglion combined with its effector organ.
The corticomedullary junction is quite easy to see. The cells of the adrenal medulla are somewhat more basophilic than those of the cortex. With appropriate stains, these medullary cells show the so called "chromaffin reaction" characteristic of cells producing catecholamine compounds, which of course is what they are doing. The adrenal medulla makes the catecholamine hormones epinephrine and norepinephrine. An example is shown below: this specimen was stained with silver to show the medullary cells as a distinct, positively-stained population.
 Recall also that the cells in the adrenal medulla have their own arterial blood supply, isolated from the supply to the cortex. In this field you see several of the large vessels supplying the medulla. While the arterial supplies are separated, the venous drainage isn't. Blood coming from the sinusoids in the cortex trickles down through the medulla and there's a common outflow, into which the hormones produced in the two regions are released.
Recall also that the cells in the adrenal medulla have their own arterial blood supply, isolated from the supply to the cortex. In this field you see several of the large vessels supplying the medulla. While the arterial supplies are separated, the venous drainage isn't. Blood coming from the sinusoids in the cortex trickles down through the medulla and there's a common outflow, into which the hormones produced in the two regions are released.
Medullary cells have a special relationship to the nervous system. Recall that neurons are secretory cells, and that a major role of neurons is export of synthesized materials: that's also the case here. The fully differentiated medullary cells have developed the secretory function of neurons to the exclusion of the conduction function, so they have no axons and structurally don't resemble neurons very much; nevertheless, they retain a good deal of their neuronal "heritage" and in particular, they retain the ability to respond to neuronal signals.
Nerve fibers from the sympathetic side of the autonomic nervous system synapse directly with the cells of the adrenal medulla, and when these neurons fire, the medullary cells respond. Instead of sending out a neural signal however, they respond by secretion—of the hormone epinephrine.
Epinephrine, of course, is well known as an actual neurotransmitter in the CNS. But more importantly, it appears to be a material for which nearly every cell in the body has receptors. Sometimes called the "fight or flight" hormone, it's released from the adrenal medulla under conditions of stress as a "wake up call" to the rest of the body that something important is about to happen (like perhaps getting killed). Its systemic and metabolic effects include decreased peristalsis in the gut, dilation of the pupils, increased heart rate and cardiac output, release of glucose from the liver, and so forth: all measures taken to put the animal in a condition to fight or flee. As one text casually notes, "Animals that have had their adrenal [medulla] removed can survive, but they are unable to respond adequately to emergencies."
The second hormone produced in the adrenal medulla, norepinephrine, has as its main function the increase of blood pressure due to vasoconstriction in the peripheral blood vessels. It too is produced in response to direct nervous stimulation. Both of these catecholamines are released via a mechanism of exocytosis, and both leave the adrenal gland in the venous outflow.
Functional Implications of Adrenal Gland Structure
It's significant that in this endocrine gland the stimulus for activity comes not via a chemical messenger from the pituitary (as in the adrenal cortex) but rather by a direct "command" from The Brain Itself. This illustrates once again the integration and complementary activities of the nervous and endocrine systems.
The most familiar example of medullary action is the "flight or fight" reaction. Imagine you are walking on the Appalachian Trail, and a large bear pops out from behind a bush 10 feet from you. Your eyes perceive the image of the bear; your brain integrates the information and decodes the image BEAR! Immediately your sympathetic nervous system, in response to the information that a B-B-B-BEAR!! is standing in front of you sends a message to the adrenal medulla; the adrenal medulla releases epinephrine into the blood. This hormone—also called "adrenaline"—has a number of target organs. It causes your gut musculature to stop peristalsis; your pupils to dilate; your circulatory system to start sending plenty of oxygenated blood to your limbs. Thus, when you turn and run as fast as you can away from that B-B-B-B-B-BEAR!!! you are putting maximum effort into the escape. (Hope it helps!)
Thyroid and Parathyroid Glands
The thyroid gland can be seen on slide 691. (Slide 691 also has a section of parathyroid gland on it.).
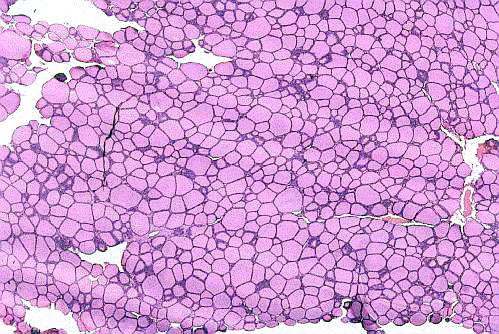 The organization of the gland into follicles is easily seen. A very low magnification image is shown at left. The appearance of this gland in light microscope preparations is unmistakable.
The organization of the gland into follicles is easily seen. A very low magnification image is shown at left. The appearance of this gland in light microscope preparations is unmistakable.
The follicles are hollow balls of cells, each with a wall composed of simple cuboidal epithelium. The amorphous material present inside the follicles is colloid, an inactive storage form of the gland's secretion. Colloid incorporates the hormones thyroxin and tri-iodothyronine as part of a large (660,000 dalton) glycoprotein, thyroglobulin.
Thyroglobulin is hydrolyzed to release the active material. The capacity to store product in an inactive form is routine in exocrine organs, but it's unique to the thyroid among endocrine glands. No other endocrine gland does this. The thyroid is unique in another way: it stores its secretory product extracellularly.
 In the regions between the follicles, CT cells of the septa which divide the
organ are present. As you would expect in any endocrine gland, the blood supply to the thyroid is
very good. There are capillary beds in all of the interfollicular spaces (these
can usually be identified by the presence of erythrocytes in the capillary
lumen, and/or by coagulated, stained blood plasma).
In the regions between the follicles, CT cells of the septa which divide the
organ are present. As you would expect in any endocrine gland, the blood supply to the thyroid is
very good. There are capillary beds in all of the interfollicular spaces (these
can usually be identified by the presence of erythrocytes in the capillary
lumen, and/or by coagulated, stained blood plasma).
C-Cells
 Also present as part of the follicular wall are the C-cells or clear cells,
sometimes called parafollicular cells. These are oval shaped, larger,
and more lightly stained than the follicular cells are. They are often hard to
identify in H&E preparations, but you should be able to find a few. The
C-cells do not produce colloid; they produce the hormone calcitonin instead.
Also present as part of the follicular wall are the C-cells or clear cells,
sometimes called parafollicular cells. These are oval shaped, larger,
and more lightly stained than the follicular cells are. They are often hard to
identify in H&E preparations, but you should be able to find a few. The
C-cells do not produce colloid; they produce the hormone calcitonin instead.
The C-cells aren't very numerous but they're important. Calcitonin plays a major role in calcium metabolism. It has the effect of lowering circulating calcium levels by stimulating uptake into the bone. Calcitonin increases the activity of osteoblasts and the synthesis of bone. It's antagonized by the hormone produced in the parathyroid gland (see below) which stimulates osteoclast actions and releases minerals from the breakdown of bone.
Vascular Relationships of the Thyroid Follicles
 Slide 691, which is stained with Masson's stain, shows clearly the outer CT capsule of the
organ and the degree of septation. The CT fibers are
stained green, and examination under high power will reveal that wisps of CT
are present in all of the interfollicular areas, providing support for the
overall structure of the gland. You may be able to make out a few erythrocytes
in the blood vessels running through the CT.
Slide 691, which is stained with Masson's stain, shows clearly the outer CT capsule of the
organ and the degree of septation. The CT fibers are
stained green, and examination under high power will reveal that wisps of CT
are present in all of the interfollicular areas, providing support for the
overall structure of the gland. You may be able to make out a few erythrocytes
in the blood vessels running through the CT.
Slide 691 also demonstrates a parathyroid gland. This is one of four small oval bodies associated with the thyroid gland, but which are separate endocrine organs in and of themselves.
 The parathyroid gland shown here has its own distinct capsule, and it's not
lobulated as is the thyroid gland. Like other endocrine organs it's well
vascularized, and all of the functional cells of the parathyroid parenchyma are
closely associated with a blood vessel.
The parathyroid gland shown here has its own distinct capsule, and it's not
lobulated as is the thyroid gland. Like other endocrine organs it's well
vascularized, and all of the functional cells of the parathyroid parenchyma are
closely associated with a blood vessel.
In most species the parathyroid gland (Pth) is embedded in the substance of the thyroid gland (T) or set into a depression on its surface, as is the case in this field. The parathyroid has its own distinct capsule (C) and blood supply from that of the thyroid, and despite its location is an independent entity. It arises from a wholly different embryonic rudiment as well.
Structurally the parathyroid hormone resembles many other endocrine glands: a cellular parenchyma of uniform appearance, cells with characteristics associated with secretion, and a high degree of vascularization. In the absence of "landmarks" (in this case the obvious presence of thyroid tissue) it would be almost impossible to distinguish it from any other giblet of endocrine tissue.
The parathyroid gland's principal product is parathormone or parathyroid hormone, usually abbreviated PTH. The metabolic effects of PTH are exactly opposite to those of calcitonin, so these two hormones are antagonists. PTH causes increased resorption of bone through osteoclast activity, and hence increased levels of serum calcium. By balancing the levels of PTH and calcitonin a normal range of calcium levels is achieved.
The most numerous parathyroid cells are the chief cells, with a round nucleus and weakly stained cytoplasm. Other types have been described but are much rarer and will not be considered here.
The detailed physiology of the thyroid and parathyroid glands is extremely important clinically, but will be dealt with in other courses. The thyroid gland's principal product, thyroxin, effects an increase in metabolic rate. In some animals under prolonged cold stress, such as Arctic wolves and sled dogs, thyroxin output is often elevated to increase the rate of body heat generation. Such dogs will sleep outdoors in weather as cold as -40°!
The calcitonin produced by the C-cells brings about a decrease in blood calcium levels, and an increase in the deposition of calcium in bone. This effect is antagonized by parathyroid hormone, which acts to release calcium from bone and increase its concentration in the blood.