THERE ARE PRE-RECORDED LECTURES FOR THIS EXERCISE
Please go to the listing page for these to download and view them
Mitochondria
Microtubules & Microfilaments
Organelles & Inclusions
Nowadays we take the concept of "cells" as the basis for living
organisms so much for granted that it's hard to realize it wasn't always this
way. The cell theory enunciated in 1838 and 1839 by Theodore Schwann
(1810-1882) holds that all living things are composed of cells, and that cells
can only arise from pre-existing cells.
Although the truth of this view is undoubted today, the concept of "spontaneous generation" of life from nonmoving precursors died very hard, and for its day, the cell theory was a daring and avant-garde concept: what today we'd call "cutting edge" science. This laboratory exercise will provide you an opportunity to examine the structure of animal cells and some of their subunits.
PRE-RECORDED LECTURES FOR THIS EXERCISE:
Basics of Cell Structure
Mitochondria
Plasma Membrane
Nucleus and Endoplasmic Reticulum
Microtubules and Microfilaments
Subcellular Inclusions
Any slide in the set will serve to demonstrate the nucleus and the cytoplasm of typical cells. Nuclear morphology varies greatly from one cell type to another, and can be used as a rough guide to cell type and activity. You'll see several different cell types in the image above. The nuclei vary in the depth and intensity of staining, in shape, and in other ways. If you examine a cell specialized for the synthesis of proteins, for example, its nucleus will differ in appearance from that of a cell with a different function. Cells with a mechanically supportive function will have nuclei nothing like secretory cells, and so on. While most cell types have only one nucleus, this isn't always the case; and the presence of more than one nucleus in a cell is another indication or either its physiological state or its nature.
It is well worth your while to get a feel for the variations of nuclear appearance in different tissues and organs, as this is of major importance in diagnosing pathologic conditions. The nucleus is one of the first of the subcellular structures to undergo changes when cellular death is imminent.
As mentioned, one pretty much universal characteristic of nuclei is that they tend to take up "basic" stains i.e, those which are alkaline in nature. Since nuclei contain comparatively large amounts of acidic material (DNA and RNA) there tends to be a very strong affinity between the nucleus and this sort of stain. This is called "basophilia" or a "basophilic staining reaction." In the case of H&E preparations, nuclei typically stain blue to dark purple, because the hematoxylin is a basic stain. Cresyl violet is another basic stain, usually used in nervous system preparations, and binds strongly to RNA and DNA.
Cytoplasm tends to be much poorer in acidic materials than nuclei (there are some exceptions to this statement) and the cytoplasmic reaction with hematoxylin (or other basic stains) tends to be markedly less than that of the nucleus. Frequently, cytoplasm is termed acidophilic or (in the case of H&E slides) eosinophilic in its staining reaction. Of course, the degree of basophilia and/or acidophilia will vary depending on cell type and physiologic state, and the terms are relative, not absolute. The image above is from the pancreas. It shows both basophilia and eosinophilia.
In this H&E preparation, the nuclei are basophilic. But while some of the cytoplasm is pink (as cytoplasm tends to be) other parts of the cytoplasm are stained the purplish-blue color of hematoxylin. What accounts for this "cytoplasmic basophilia?" As with nuclear basophilia, cytoplasmic basophilia is due to a high concentration of acidic materials. In this case it's the ribosomes of the rough endoplasmic reticulum. Ribosomes are made mostly of RNA, which has the same affinity for basic stains as any other RNA or DNA.
This staining pattern is important and it tells you something about what these cells are doing. Pancreatic cells of this type secrete digestive enzymes: the eosinophilic granules/cytoplasm at one end are the proteinaceous secretory product. The basophilic areas of cytoplasm are where these enzymes are being made. This "cytoplasmic basophilia" results from the presence of large amounts of RNA in the RER, which is clumped at the basal region of the cell. The apical eosinophilic areas are relatively free of RNA, and contain the secretory granules of peptides that will eventually be released. Hence the disparity of staining.
The presence or absence of basophilia is an important clue to what a cell's doing, and one of the first things to look for in examining a preparation, especially one made with H&E. While cytoplasmic basophilia is the norm in a few cell types (such as this one) it's decidedly not normal in others, and may be a sign of unregulated cell division.
A certain amount of DNA will bind a certain amount of any given basic dye. But as a general rule, the actual appearance of the stained material or structure in microscope sections depends on how thick the stain is. The reason for this is physical: stains impart color by absorbing light, and the more stain there is between you and the light source, the more heavily stained a structure will appear. Some types of cells are specialized as large scale protein producers (the pancreatic cells just examined are a classic example) and their cytoplasmic staining and nuclear morphology reflect this. Since the DNA of these cells is uncoiled (for access during protein synthesis) the staining reaction in these regions of the nucleus is less pronounced. The DNA still absorbs the stain, but the stained material is dispersed throughout the entire nuclear volume. Uncoiling of the DNA disperses the stain molecules over a larger area, they aren't "stacked up" on top of each other. Hence they don't absorb so much light per unit thickness of the section, and thus the apparent intensity of staining is reduced. Nuclei with uncoiled DNA and with spotty or absent staining are described as "vesicular." You also expect to see dense nucleoli in these vesicular nuclei. The nucleolus is very strongly basophilic, because it's made almost entirely of RNA.
As an example of this, compare the two images above. The one on the left is of a lymphocyte, one of the circulating white blood cells (see Exercise 6). The one on the right is a large Purkinje neuron in the cerebellum (see Exercise 9). In circulation the lymphocyte is in an inactive state; its nucleus is very dense, because the DNA has been coiled up into its storage configuration. There is a minimal amount of cytoplasm, visible here only as a thin crescentic rim. Densely packed DNA is sometimes called "heterochromatin" and such nuclei are termed "heterochromatic."
In the neuron there's active secretion going on (neurons make neurotransmitters in large quantities). Hence the nucleus is "vesicular," or pale-staining. This term implies one in which large amounts of euchromatin are present. Euchromatin is the antithesis of heterochromatin: the nuclear DNA is unwound for reading and there is virtually no staining visible. Such a nuclear appearance is typical of cells actively engaged in secretion, and most especially protein secretion. This cell is a neuron—and neurons are usually very active in this respect. A cell such as this is said to have a "euchromatic" nucleus. Remember that exactly the same DNA is present, assuming both cells come from the same animal. But the unwound DNA is less tightly packed, so the nuclear region is not dense, but pale. Note that in other cells of this image, the non-neuronal supporting cells, the nuclear configuration is that of relatively quiescent cells with little if any secretory function.
The nucleolus, also indicated here, is the site of mRNA synthesis. Its very densely concentrated, rich in mRNA, and appears as a dark, almost perfectly circular, dot in the middle of the unstained nuclear region. The mRNA is present in large amounts to facilitate the protein-making activity. A very prominent nucleolus (or even more than one) is another sign of secretory activity.
As a first approximation when you are attempting to identify a cell type, the combination of a basophilic cytoplasm and a vesicular nucleus sporting prominent nucleoli is a pretty good clue that the cell in question is secretory.
Most subcellular organelles and inclusions are visible with the light microscope, but unfortunately, routine H&E staining is usually insufficient to visualize these structures. Special procedures must be used, but don't expect them to look like H&E stained material; the purpose of such staining routines is not to reveal general morphology, but to take advantage of some chemical peculiarity to make a particular structure or structures visible. Even with special routines, the level of detail visible in the LM is not too high, and the electron microscope is needed to get resolution high enough to make out the internal structure of, say, mitochondria.
Much to many people's surprise, mitochondria are visible in the light
microscope. It requires a special stain to make them visible. Mitochondria are
very small, on the order of 0.1 to 0.2 mm in diameter, actually about
the size of bacteria. Hence they are at the limit of resolution of even very
good light microscopes. We are much more used to seeing images of them as they
appear in the electron microscope. This transmission EM picture shows mitochondria clearly. They have the classic appearance of double walled oval structures, with internal cristae for the amplification of surface area. (The long snake-like structures in among them are invaginations of the plasma membrane.) The cytoplasmic matrix is significantly denser than the cytoplasm of the cell, and the dark dots inside the mitochondria are mitochondrial granules, condensations of calcium ions that are fairly commonly seen. The activity of mitochondria is dependent at least in part on ion flux and the movement of charged particles across the inner and outer membranes.
Mitochondria are a very nice example of a structure whose true nature was appreciated only after the introduction of the electron microscope. They were known to exist as early as 1890, because with special stains they can be seen with a light microscope. They're so small, however (about the size of bacteria) that in an LM they appear as little more than dark dots. Most people visualize mitochondria in terms of the image above from an electron microscope. What isn't generally realized is that mitochondria are visible in the light microscope with special stains, as you see in the image to the right. They respond well to the stain Janus Green (used in the image here). The problem is, that mitochondria are very small, about the size of bacteria. The tiny black flecks in this image indicated by the arrows, are the mitochondria, all of perhaps half a micron or so long.
Mitochondria were discovered by a German biologist, Robert Altmann, in the 19th Century. Altmann called them "bioblasts" and believed they were the fundamental units of living things. In this he was not so far off from the truth, but the techniques of his day were pretty crude and he died without ever having proved his theory. The term "mitochondrion" was coined by Christian Benda about 1895, and is Greek for "thread granules," a pretty fair description of their morphology.
By the 1920's and 1930's it was clear what their function was, but they remained "black boxes" which consumed oxygen and produced ATP, but nobody had a clue to how they worked. In the mid-1950's, the electron microscope's high magnification and resolution made it possible to look inside the box, to see how it was built, and to formulate testable hypotheses about how it functioned. As with so many other aspects of the life sciences, the current picture of the mitochondrion and its function is built on fundamental observations made with the electron microscope. The drawing at left is from a 1953 paper by George Palade (right) showing his concept of the internal structure. Palade won the Nobel Prize for his work, and the elucidation of the inner details of the mitochondrion was one of the very early triumphs of the new technique of electron microscopy. In the decades between 1945 and 1970, the EM revolutionized the life sciences. It created a whole new discipline, what today is called cell biology. Everything modern students are taught about cells, cell physiology, histology, and tissue architecture—all of it, including genetic engineering—rests on the foundation laid by the early electron microscopists of the 1940's and 1950's.
Ribosomes
Ribosomes and rough endoplasmic reticulum (RER) are not individually visible in
the light microscope, but large aggregations of them show up in the form of
basophilic regions of cytoplasm. The basal basophilia of the pancreatic acinar cell is an example of this phenomenon.
Ribosomes in the form of RER are most easily seen in nervous tissue. In the large cell bodies of neurons, the rough endoplasmic reticulum takes up basic stains like cresyl violet quite readily; the RER is visualized as large flakes or patches. This example shows these so-called Nissl bodies in the stellate soma of a neuron, as a coarse granularity in the cytoplasm surrounding the nucleus.
The term "endoplasmic reticulum" was coined by Keith Porter in the 1950's to describe its appearance in the transmission EM: an aggregation of flattened sacs and tubules forming a network in the basal regions of the cell. The current concept of the nature and function of RER is based on the images he and other microscopists of the period produced. Porter later demonstrated that the basal cytoplasmic basophilia of the pancreatic cells and the RER were one and the same thing. This light micrograph is stained with cresyl violet. The acidic, RNA-rich ribosomes of the RER bind it in large amounts. In neurons the secretory activity is very high, so neurons have vast amounts of RER; enough to form LM-visible clumps in this type of preparation. This image is from slide 552.
Cilia are very common in mammalian systems, and are most easily seen in slides of the respiratory system. Of course, ciliated cells aren't restricted to mammals or to the respiratory system. They were first described from the reproductive tracts of birds (by Purkinje and Valentin in 1831) and are present in all of the metazoa.
This example of cilia (left) is from slide 26, the trachea from a dog. This organ
is lined with ciliated cells. The cilia can be seen projecting into the lumen
as a sort of "fringe" whose function is to trap inhaled dirt
particles and sweep them back up to the pharyngeal region. Similar cells can be
seen in some areas of slide 115. In the electron microscope cilia have a very characteristic appearance, quite unmistakable for anything else when they're cut in cross section. The center of the cilium has two large single microtubules, and around its periphery are several paired "doublets" of microtubules in a wheel-like pattern.
Microvilli are long finger-like projections of cytoplasm. Microvilli are to be expected wherever it's necessary to increase surface area without increasing cell size, especially in regions of absorption, such as the intestine and kidney.
Above at right you see an example from Slide 40. The microvilli aren't individually resolvable with the LM; instead they appear as a broad refractile "brush border" on these absorptive cells. this brush border can be seen in more detail in the electron micrograph at right. The microvilli are all about the same length, and there's a cluster of mitochondria in the region of the cell just below them.
Microvilli can be confused with cilia, but they're much smaller, and they lack the internal component of microtubules. It's easy to distinguish the two in an electron microscope, and in the light microscope the best guide is their size relative to the cell the "decorate." These two images were taken at the same magnification (about 1500x) but it's easy to see that in the picture at left the cilia are a much larger proportion of the height of the cells than the brush border at right is. Also, cilia are large enough to see individually and microvilli aren't. An electron micrograph of the two side by side (left) leaves little room for doubt about their relative size.
The Plasma Membrane
The plasma membrane, the limiting barrier between a cell and the outside world, is not directly visualizable with the LM, but its presence can be deduced from the fact that there is demonstrably an "inside" and an "outside" for any given cell. Although the concept of the membrane was accepted from the mid 19th century onwards, it was not in fact until the 1950's that the EM made it possible to demonstrate it as a physical entity.
The Glycocalyx or Cell Surface Coat
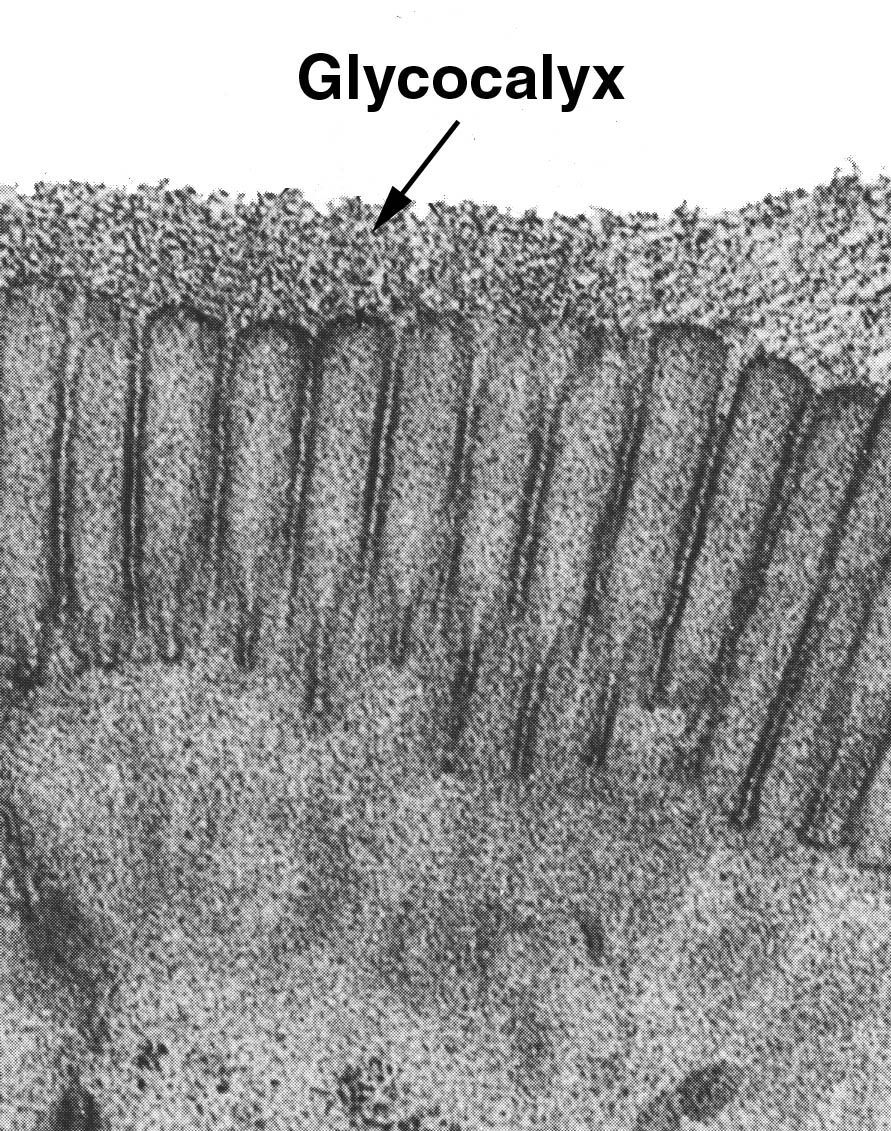 Some special stains for carbohydrate will reveal a cellular component which strictly speaking is neither an
organelle nor an inclusion, but falls into the category of plasma membrane
specializations. This is the glycocalyx or cell surface coat. All cells
are covered on their outside surfaces by a polysaccharide material they
secrete, which is bound to the outer leaflet of the plasma membrane. This layer
acts as an ion trap, and helps the cells maintain normal internal levels of
ions; it also serves a protective function. A demonstration of this is
available.
Some special stains for carbohydrate will reveal a cellular component which strictly speaking is neither an
organelle nor an inclusion, but falls into the category of plasma membrane
specializations. This is the glycocalyx or cell surface coat. All cells
are covered on their outside surfaces by a polysaccharide material they
secrete, which is bound to the outer leaflet of the plasma membrane. This layer
acts as an ion trap, and helps the cells maintain normal internal levels of
ions; it also serves a protective function. A demonstration of this is
available.
The image at left, a transmission electron micrograph, shows the glycocalyx of the intestinal cells of a cat. All cells have a glycocalx, but it happens that this particular site is provided with a very thick and easily demonstrable one. The word "glycocalyx" was coined to indicate the nature of this cell surface coat: it translates from the Greek as "sweet husk," an appropriate term for a material mainly composed of carbohydrates. It's hard to see in the light microscope, though it can be made visible with stains like the PAS method.
It has many functions. It acts as a protective coating for cells exposed to deleterious environments (as is the case in the intestine) and also as an "ion trap" to mediate the steepness of the ionic gradient between the intra- and extra-cellular environments. In the intestine it is also thought to have some degree of enzymatic activity, and actually participate in digestion. The glycocalyx is made by the cells on which it's located. It may be supplemented by the output of glands and goblet cells, but it's always to some extent made locally.
Picture credit: Ito, S. 1965. The surface coat of enteric microvilli. Journal of Cell Biology 27:475
Microtubules are organelles. One place they're found is in the core in cilia and
flagellae, but while cilia and flagellae are easily visible in the LM, the
microtubules inside unfortunately are not. An electron microscope is used
demonstrate their basic structure. This slide
shows mitosis in whitefish eggs. In the mitotic process, spindle fibers are
formed connecting condensed anaphase chromosomes to anchor points in each
forming daughter cell. These spindle fibers are microtubular in nature. The
whitefish embryo grows rapidly and mitotic figures are numerous. Microtubules are composed of polymerized subunits of a special protein, tubulin. In the case of spindle fibers, the fibers are formed, and as division proceeds, they are depolymerized by removal of subunits at the end furthest from the chromosomes. Eventually the entire set of tubules has been dismantled. When division occurs, the tubules are reassembled and disassembled again. If you locate a cell whose chromosomes are lined up in the center, or just beginning to separate, you should be able to make out the microtubules of the spindle fibers. These appear to radiate from two points, one on each side of the about-to-divide cell.
Microtubules have a vital role even in cells that don't divide. They're part of the so-called "cytoskeleton." Many cells have irregular shapes (neurons, for example) and the microtubules in the cytoplasm act to maintain the normal geometry. Microtubules are produced internally by the same sort of synthetic machinery that makes other cellular components. Like scaffolding, they can be "dismantled" and re-assembled as the cell's needs change. Many cell types have only a temporary demand for microtubules, and the cycling of materials through the pathways is controlled by internal and external signals.
Slide 54 (shown above right) provides a good example of another microtubular structure. In cells actively undergoing mitotic division, such as those you see in the image here, microtubules (MT) are involved in the separation of chromosomes during the karyokinesis phase. In the center of this field the cell's chromosomes (C) have arranged themselves on the equatorial plate and microtubules can be seen connecting them to the centrosomes at the opposite ends of the cell. The old term for these particular microtubules was "spindle fibers." Upon depolymerization, they will move the two sets of chromosomes apart.
Microtubules are involved in cellular movement. Cilia and flagellae have a core of microtubules in a highly-conserved arrangement that causes them to move. This in turn can propel the entire cell forward (as, for example, the flagellum on a spermatozoon does) or move sheets of fluid (as in the respiratory or reproductive tracts).
One organelle which was the source of much controversy is the Golgi apparatus, named for its discoverer, Camillo Golgi. The Golgi apparatus (out of respect for the man, not "golgi" with a lower-case "g") is intimately involved in the chemical modification of proteins synthesized in the RER (usually by adding sugars to them) and with their "packaging" into membrane bound vesicles for transport to the surface of the cell and release.
The Golgi apparatus, as you might expect, is very active and most easily visible in cells that are secreting materials rapidly. The appearance of this organelle is another clue that the cell is secretory in nature. One place it's relatively easy to see this organelle is in the cells lining the epididymis, that portion of the male reproductive tract in which sperm are matured and made capable of fertilizing oocytes. The epididymis is a secretory organ, producing glycoproteins that coat the sperm.
On slide 241 you'll see the Golgi apparatus as a clear area near the nucleus of the cells lining the organ. These slides are stained with H&E, so the unstained Golgi apparatus stands out from the background. The Golgi apparatus stains poorly with H&E because it has neither an acidic or a basic nature; but because of its high content of sugars, it can frequently be rendered visible with the PAS stain for carbohydrates. Glycoprotein production involves the Golgi apparatus: its glycosylation enzymes attach the sugar moieties to the protein backbone made in the rough endoplasmic reticulum. Consequently, the cells lining the epididymis have a very well-developed Golgi apparatus, and the same is true in most other cases of cells with similar functions. You see the GA here as clear areas on the luminal side of the nucleus of the columnar epithelium. The lumen of this organ is filled with spermatozoa, whose basophilic heads are seen as dark dots. In a PAS stained preparation, the Golgi apparatus often takes up the stain because of the carbohydrates present within it.
Here's an example of the Golgi apparatus as it is seen in the electron microscope. The complex arrangements of stacked cisternae, with their associated vesicles, is obvious. The Golgi apparatus has a convex forming face (at the bottom of this image) to which proteins to be packaged and/or modified are brought; and a concave maturing face from which the vesicles containing the modified product are pinched off.
For many years, the existence of this organelle was controversial, and it was considered by some people to be an artifact of preparation; but its demonstrability in unstained preparations (using phase contrast microscopy) and in the transmission electron microscope settled the question. The Golgi apparatus is found in most cell types, though it's sometimes inconspicuous.
Subcellular inclusions are nonmoving material that are usually the result of metabolic activity of the cells in which they're found. In most cases they require special stains to be seen clearly. They may be pigments produced in the cell, or they may be accumulations of nutritive materials such as fat or carbohydrates.
Lipofuscin pigment, often called "wear-and-tear" pigment, is easily seen in nervous tissue and in areas of high macrophage activity. Lipofuscin is the undigested residue of subcellular lytic reactions. As organelles become aged and useless, they are broken down for their components, and what is left that can't be salvaged is the pigment you see here. It tends to be greater in older animals. Since neurons do not divide, they tend to accumulate the pigment over the years. Hence the name "wear-and-tear" pigment. Lipofuscin is also found in fat cells, and it's the material which turns the fat of older animals yellow.
Macrophages are scavenger cells, and their job is to pick up and destroy potentially harmful materials, such as bacteria, dust particles, etc. They also are called in to clean up an area of infection after other immune system cells have dealt with it. Macrophages can contain lipofuscin from almost any source: dead cells, their own organelles, killed bacteria, and so forth. They may also contain the pigment that's produced by the breakdown of old red blood cells, hemosiderin (see below).
These images are from slide 27. At left is an example of cells containing lipofuscin, the indigestible residue of the breakdown of cellular material. In this field you see some macrophages, the scavenger cells of the body, at work. They have entered an area in which infection has taken place and are working to remove the dead cells and/or bacteria in the region. The lipofuscin is the brownish-gold pigment visible inside them. Had there been hemorrhage in this region, some of the macrophages would have contained hemosiderin, too. In H&E sections it can be difficult to determine whether the pigment seen is lipofuscin or hemosiderin, but and easy way to distinguish the two is by using a stain for iron (such as Prussian Blue) that gives a positive reaction with hemosiderin. There are some special stains for lipofuscin, but once you gain some experience with seeing it in H&E slides it will be easy to identify, based on knowledge of the cell type and activities. In the higher magnification image at right the nature of the lipofuscin inclusions in the macrophages is easily seen.
The granular appearance of the inclusion, and its presence in some cells but not others, is a clue to its nature and origin. Lipofuscin inclusions are formed by the fusion of a primary lysozome and a phagocytic vesicle. The combined inclusion vesicles will be of varying size, and also of varying density. Note also that the nucleus of the large macrophage in the upper left corner of the image is pretty well obscured by the lipofuscin inclusions. This is a very active cell. Newly-recruited macrophages just differentiating in the region would have fewer granules in them. The density of the inclusions and their distribution will also vary somewhat depending on what it is the macrophage has engulfed and what's being broken down in the cytoplasm.
Glycogen
Glycogen is another type of pigment inclusion. Glycogen is a polymer of glucose, used as a storage material when glucose supplies are high. The liver and the muscles store large quantities of it to release on demand by depolymerizing it back to glucose. You'll see it on slide 500, from which this image was made. This is liver, stained with the periodic acid-Schiff's reaction (PAS). The PAS reaction turns carbohydrate constituents a magenta color; the liver stores a lot of glycogen and it's seen here as coarse magenta-colored granular deposits. Unlike lipofuscin, glycogen is not exclusively located in one part of the cell. In those cells which accumulate it, it will be usually found scattered through the cytoplasm.
Here's another example, this one from a demonstration slide not in your set. This is skeletal muscle, also stained with PAS. The interior volume of a skeletal muscle cell is packed with contractile units, leaving very little space for anything else. There's typically a good deal of glycogen inside these cells, used as a back-up energy source. In this image the glycogen has been "squeezed" into one corner of the cells and accumulates as magenta-colored pools of material (arrows).
Melanin is yet another pigment inclusion. It's the most common of the biological pigments, and is found, so far as I'm aware, in virtually every phylum of the animal kingdom. It's produced by special cells derived from the neural crest, the melanocytes. These cells migrate during development to specific places, mainly in the integument, but also in some parts of the nervous system.

Melanin pigment is responsible for part of the coloration of skin and hair. The melanocytes are residents of the deepest layer, and pass the pigment they make on to other cells, that in turn carry it to the surface. It's produced via a synthetic pathway that uses the amino acid tyrosine as a raw material. Lack of an enzyme in this pathway can occur due to a spontaneous and fairly common mutation. When this enzyme is missing or inactive, the pathway is blocked, so no melanin can be made. The inability to synthesize melanin is the condition we call albinism; an true albino animal has white fur or feathers and no pigmentation in its eyes, either. The reverse condition (often seen as a color phase variation in rodents and some carnivores) is melanism, in which more than the usual amount is made. Such animals are said to be melanistic. The black squirrels found in the streets and parks of Washington DC are a nice example. They are melanistic strains of the common grey squirrel, Sciurus carolinensis and freely interbreed with greys to form a range of color variations from "normal" to deep blue-black.
You can see melanin on slide 24. These pictures show melanin as a dark brown material in the deep regions of the skin of the footpad. granular material in cells at the junction of the epidermis and the underlying connective tissue. The image on the right has melanocytes visible as unstained cells in the deepest layer of the skin. The melanocytes don't contain much melanin. Instead of retaining the pigment they pass it along to the adjacent keratinocytes.
Melanocytes are a stable and long-lived population of cells. The normal life span of melanocytes is years, probably in most cases the entire life of the animal. As with other normally non-dividing cells, however, sometimes things go haywire. If melanocytes do start dividing, it's a very serious matter, as they lack the ability to control their division. The uncontrolled growth can become highly invasive and move into surrounding areas. The worst case is a malignant melanoma, a particularly metastatic and often fatal type of cancer. Exactly what causes it is unknown, but well documented risk factors include overexposure to the sun and physical damage to a pigmented wart or mole.
You may encounter melanin in neurons as well.
Hemosiderin is the indigestible residue of blood cell destruction, and hence the "hemo" in the word refers to hemoglobin, the blood's oxygen carrying pigment. The heme moiety of hemoglobin contains an iron atom at its core, and so staining routines for hemosiderin rely on detection of iron. Hemosiderin is most easily demonstrated in the spleen, where aged erythrocytes are phagocytosed; but it can also be found in hemal nodes and in the liver.
Hemosiderin can be seen on slides 673 and 674. The image above is from slide 674. It's stained with Prussian Blue and counterstained with neutral red. Because reclamation of erythrocytes takes place in the spleen, hemosiderin tends to accumulate in large amounts in the resident splenic macrophages. You can compare the staining reaction here. The hemosiderin is visible as a deep blue pigment, in very large clumps. The clumps are actually macrophages that have engulfed large numbers of erythrocytes. Smaller and less-intensely stained clumps of blue represent cells with less hemosiderin in them. The lipofuscin, not having any iron in it, doesn't stain with the Prussian Blue routine, and appears as its own brown-gold color. In slide 673, stained with H&E, the two pigments are more or less indistinguishable except for the size of the deposits. This is a classic example of differentiating morphologically similar structures by their chemical differences, i.e., histochemistry in action.
How Do You Tell What It Is?
We've now looked at three of the most common intracellular pigment inclusions, and invariably I'm asked how to tell them apart. The best answer is "special staining," i.e., the Kluver-Barrera stain for lipofuscin, the Fontana-Masson stain for melanin, and the Prussian Blue stain for hemosiderin will always allow you to differentiate these three. As a practical matter, however, experience is almost as good a guide. If you know the cell type and tissue type you're working with, one or another can be ruled out: you wouldn't expect to find melanin in the intestinal absorptive epithelium, and you would be very surprised to see lipofuscin in it, either. With time and experience and understanding of different cell types and their functions, this will become pretty much the way you do it.
That said, however, if you have any doubt, and the matter is of some urgency, you shouldn't hesitate to use special stains. If you were working at NASA and examining tissues from the first extraterrestrial organism brought back by some space expedition, you'd definitely want hard evidence of what the "brown stuff" is and isn't. Send it to the histopath lab and have all three routines done.
Lipid is usually a nutritive inclusion, one that has a role as an energy
source in the metabolism of the cell. The time of residence of lipid in a cell
may be very short (minutes to hours, as in absorptive cells of the gut) or it
may be much longer (days to months, as in adipose tissue). Inside the cell it's
stored in vacuoles that are usually easily seen with the light microscope, but
are much better appreciated in the EM. If lipid metabolism is interfered
with—there are some genetic defects in which this occurs—a lipid storage
disease may result, in which lipid accumulates in abnormal locations or
in abnormal amounts. Lipids are normally lost in processing tissues for wax embedments: the solvents used to remove the wax also remove the lipid, and the droplets are seen as open spaces. The best way to visualize lipids is in tissues that have been frozen and cut with a freezing microtome (a cryostat). The usual stains are Oil Red O and Sudan Black. Examples of both are seen here, Oil Red O on the left and Sudan Black on the right. Both are unfixed, frozen specimens taken as biopsy samples during surgery.