|
Vol.
59 (3) 2004 |
INVESTIGATION OF THE FIRST TRANSMISSIBLE GASTRO-ENTERITIS (TGE)
EPIDEMIC IN PIGS IN ISRAEL
Brenner, J.1, Yadin,
H.1, Lavi, J.4, Perl, S.2, Edery,
N.2, Elad, D.3, Bargut, A.4, Pozzi,
S.4, Lavazza A.5, and Cordioli P.
5
1. Division of Virology and Preventive
Neonatal Diseases Unit,
2. Pathology and 3. Bacteriology, Kimron
Veterinary Institute, 50250, Bet Dagan,
4 . Freelancer Veterinary
Surgeon, Israel and 5. Istituto Zooprofilatico Sperimentale della
Lombardia e dell’Emilia-Romagna “Bruno Ubertini” Via Bianchi 9, Brescia
(BS), Italia. |
|
Abstract
Transmissible gastro-enteritis (TGE) is a
contagious disease of pigs that occurs as explosive epizootics. This
communication reports on the evidence that we gathered in order to confirm
the first epidemic of TGE in Israel and external validation corroborated
our clinical suspicion and laboratory findings.
 Our Discussion forum is open for
your comments on this article Our Discussion forum is open for
your comments on this article
|
Materials
and methods Results
Discussion
back to top
Introduction
Transmissible
gastro-enteritis (TGE) is contagious disease of pigs that occurs as explosive
epizootics. TGE is caused by the TGE virus (TGEV), a member of the
coronaviridae. A distinct respiratory variant the Porcine Respiratory Corona
Virus ( PRCV) has been recognized since the 1980’s, a deletion mutant of the
TGEV (1). Two other coronaviruses antigenically distinct are the Porcine
Epidemic Diarrhea Virus (PEDV) and the Hemagglutinating Encephalitis Virus (HEV)
(2).
TGEV is a common cause of
diarrhea in pigs, affecting all ages but significant deaths only occur in
suckling pigs, with the severity related to the age of the animals infected.
Almost all susceptible piglets under 10 days of age die within few days of
exposure, but the mortality decreases the older they become. Only mild signs
such as vomiting, regurgitation and agalactia are present in the lactating dams.
In an endemic situation, namely, in vaccinated animals and/or where the acute
wave has faded, sporadic diarrhea in older animals and post-weaning animals in
contaminated nurseries are the spare clinical evidences of TGEV infection. As a
member of the coronavirus group, TGEV is primarily an enteric virus, destroying
enterocytes of the small intestine (Fig. 1), causing villous atrophy.
Extra-intestinal sites of virus multiplication include the respiratory tract and
mammary tissues (3,4,5), but the virus is most readily isolated from the
intestinal tract and feces.
The first report of clinical
disease in pigs caused by coronaviruses dates to 1946 (6) and TGE occurs
throughout the world. The emergence of PRCV coincided with the disappearance of
TGEV in Europe (7). In mid 1980’s, a previously unrecognized porcine coronavirus
spreading through Europe was identified (8). The name porcine epidemic diarrhea
virus (PEDV) was adopted (9). At present, PEDV has been identified in most
swine-producing countries, except in the Americas (9).
When the TGEV spreads within
a fully susceptible herd with no previous history of TGEV infection, up to 100%
mortality is expected among newborn pigs, showing marked diarrhea and
dehydration in weaned pigs, while inappetence, vomiting and
diarrhea are typical signs in adult animals. Partial or total agalactia of sows
is common (5,9).
The Israeli pig industry benefits from a particular
epidemiological situation. Due to religious customs in the middle east, where
the Jewish and Muslim religions prevails, the pig industry is largely isolated,
and far away from any zone with intensive pig industry. Consequently, very few
epidemics have been recorded among the Israeli pig herds. This communication
reports on the first epidemic of TGE in Israel. The investigation was carried
out pursuing diagnostic methodology of emerging diseases.
Introduction
Results
Discussion
back to top
Materials and Methods
Case
description
The first cases of diarrhea followed by dehydration and
death of newborn piglets, 1 to 7 days old were noted in one pigsty in northern
Israel on May 7th 2004 and the episode gained epidemic proportions, with
mortality as high as 70 to 80% during the first week of life and proportionally
less in convalescent groups of piglets. The area where this outbreak occurred is
nicknamed the “swine hill” which is located near Evlin. This hill is about 1.5
by 1.5 kilometers. Thirteen pig herds are concentrated in this restricted zone.
One to two days later after the first outbreak, other herds reported the same
clinical signs. Additional clinical signs that accompanied this outbreak were
vomiting and regurgitation and a partial agalactia in lactating sows. Two
piglets of 2 days old were brought to the Kimron Veterinary Institute (KVI) for
post mortem examination. The histological examination of the small intestine
revealed typical (4,9) villous atrophy, suggesting an enteric virus infection.
On May 24th a group of investigators from the Kimron
Veterinary Institute paid a visit to one of the affected farms. Upon the visit,
the clinical manifestations appeared as reported by the local practitioner. The
followed materials were collected on the farm: 5 individual diarrheic feces
samples, 5 moribund piglets, 6 blood samples with an anti-coagulant (3 of
diseased and 3 of convalescent animals, respectively) and 10 blood samples
without anti-coagulant (5 from diseased animals and 5 from convalescent ones,
respectively). Four of the 5 moribund animals arrived alive and one died during
transport. They were sacrificed immediately upon arrival and the feces and
intestinal segments as well as biopsies of all the internal parenchyma and
brain, were prepared for histopathological examination. An additional two sets
of the same tissues were store at -20oC, for further analysis. One
set of intestinal segments from the 5 piglets were shipped to the Istituto
Zooprofilatico, (IZS) Brescia, Italy.
One week later
another batch of diarrheic feces arrived to KVI from newly infected herds. This
batch comprised nine pooled feces; each sample represented one infected
litter.
Antigenic identification
Assuming that if
porcine and bovine coronaviruses share common antigenic epitopes, we used the
bovine assays for the diagnosis of rotavirus and coronavirus (Bio-X Combined
Digestive ELISA Kit, for antigenic diagnosis in bovine faeces of rotavirus,
coronavirus, E coli K99, B-6900 Marche-en-Famenne, Belgium) according to the
manufacturer’s instructions.
Another set of antigen diagnosis was carried
out at the IZS, using the immuno-fluorescence (IFA) on intestinal sections,
electron-microscopy (EM) and immuno-EM (IEM) with specific anti-TGEV and
polymerase chain reaction (PCR) (Kim et al., 12), and virus isolation.
For the IFA and for the IEM assays performed on the
intestinal sections, hyperimmune serum specific for porcine coronavirus was
used.
Serology
This assay was performed at the IZS in Brescia,
using a commercial kit ELISA (INGENASA) for the presence of anti-TGEV antibodies
in infected and convalescent animals.
Necropsy
All the parenchyma samples were processed
regularly and were prepared for staining with H&E on samples embedded in
paraffin.
Hematology
Biochemical and hematological profile was
done on all the blood samples.
Bacteriology
The routine laboratory diagnostic
procedures were used as described elsewhere (10).
Introduction
Materials
and methods Discussion
back to top
Results
Antigenic discovery
|
In total 63% of the fecal samples were positive
for bovine corona virus antigen(s) (6/10 and 6/9 for the first and second
sets of samples, respectively).
All the assays combined together, namely, EM and
IEM, PCR and IFA (Fig. 2) performed at the IZS in Brescia, showed that in
all the 5 samples TGEV was present although none of the assays alone could
identify the TGEV in all the samples (Table 1). Virus
isolation succeeded in two out of three diseased piglets. This isolation
attempt was performed on piglets originating from a farm on day 45 of the
ongoing TGE epidemic (Lavazza and Cordioli, personal
communication).
Serology
The sera derived from
diseased animals had a very weak ELISA anti-TGEV reaction while the
convalescent sera showed a very strong anti-TGEV reaction.
Necropsy
Villous atrophy
was noted, suggesting an acute enterovirus infection (Fig. 1). Massive
infiltration of inflammatory cellular components, vacuolization of
enterocytes and destructive process were all sequelae of the ongoing
infection. |
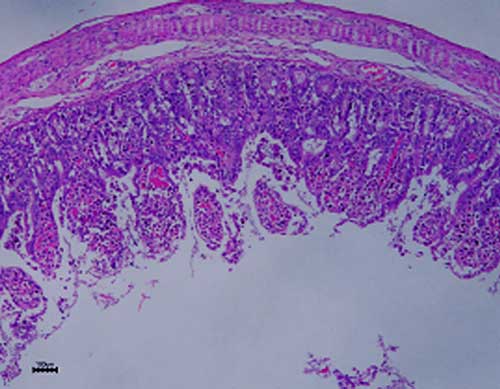
Figure 1. Villous atrophy and vacuolization of enterocytes
with a massive infiltration of inflammatory cellular
components. |
|
Hematology
The WBC, lymphocyte and
neutrophil counts are presented in Table 1.
As seen in Table 2, the hematological profile of
the diseased animals shows a remarkable lymphopenia and a moderate
neutrophilia. The L/N ratio range is a characteristic haematologic picture
of acute viral attack.
Bacteriology
All the
bacteriological assays resulted negative for the presence of
enteropathogenic bacteria including Clostridium perfringens type C, the
causative agent of porcine enterotoxemia in piglets. |
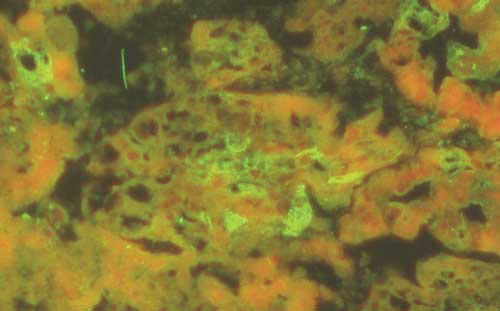
Figure 2. Immunofluorescence
staining of the small intestine with monoclonal anti-porcine corona virus
antibody.
|
Table
1 -
A Summary of the PCR, ME, IEM and IFA results from the IZS
laboratories .
|
Animal
Number |
TGEV-PCR |
ME |
IEM |
IFA
pools |
|
1 |
+ |
- |
- |
|
|
2 |
- |
+ |
+ |
|
|
3 |
- |
+/- |
+ |
+ |
|
4 |
- |
+ |
+ |
|
|
5 |
+ |
+ |
+ |
+ |
Table
2.
Range of White blood cells, lymphocyte and neutrophil counts and ratio N/L of
diseased and convalescent animals
|
|
WBC |
Lymphocytes |
Neutrophiles |
L/N* ratio
range |
|
Diseased
|
9-14.2x103
|
3.1-4.5x103 |
3.8-7.6x103 |
0.3-0.5/1
|
|
Convalescent |
11-17.8x103 |
9.7-11.5x103 |
0.8-4.6x103 |
3 to
10/1 |
*lymphocyte/neutrophil
ratio.
Introduction Materials
and methods Results
back to top
Discussion
The Israeli intensive pig industry is
isolated from other areas of farming. Moreover, it was maintained in
quasi-closed premises, 13 pig herds in one region, with no other industrial
pigsties within approximately 100 km. Due to this particular situation, Israeli
Veterinary Services and KVI lacked species-specific diagnostic tools for the
diagnosis of specific swine diseases. Nevertheless, we have diagnosed TGEV
antigens in relatively short time by using non-species specific diagnostic kit.
A specialized laboratory (IZS) that deals regularly with swine diseases has
found the TGEV in the same samples, reconfirming our primary results.
The differential diagnosis (11) of enteric
neonatal diseases deals with many possible causative agents.
The typical findings of villous atrophy
(Figure 1) and lymphopenia (Table 2), pointed toward a probable viral (acute)
attack. The clinical manifestations especially the age distribution of the
diseased animals at risk, the rapid spread of the disease among the pigsties,
its high mortality rate, the antigenic ELISA positive reaction in 63% of the
fecal samples submitted to KVI and the confirmations tests from the IZS, lead us
to the conclusion that TGEV is responsible for this episode.
Whether the virus originated from bringing
or smuggling of infectious material from abroad, or from the wild boar
population is a matter of speculation.
Introduction Materials
and methods Results
Discussion
back to top
LINKS TO OTHER ARTICLES IN
THIS ISSUE
References
1. Pensaert, M.B. and DeBouck, P.
and Reynolds, D. J. An immunoelectron microscopic and immunofluorescent study on
the antigenic relationship between the coronavirus-like agent CV777 and several
coronaviruses. Arch. Virol. 68: 45-52 1981.
2. Transmissible gastroenteritis.
In: O. I. E. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals
Fifth Edition, 2004, pp 792-801.
3. Kemeny, L. J., Wiltsey, V. L. and Riley,
J. L. Upper respiratory infection of lactating sows with transmissible
gastroenteritis virus following contact exposure to infected piglets. Cornell
Vet. 65: 352-362 1975.
4. Cooper V.L. Diagnosis of neonatal pig diarrhea.
Vet. Clin. North Am. Food Anim. Prac 2000.
5. Prithchard, G.C. Transmissible
gastroenteritis and porcine epidemic diarrhea in Britain. Vet. Rec. 144:
616-6181999.
6. Doyle, L. P. and Hutching, L. M. A transmissible
gastroenteritis in pigs. J. Am. Vet. Med. Assoc. 108: 257-259 1946.
7. Wood,
E. N. An apparently new syndrome of porcine epidemic diarrhea. Vet. Rec. 100:
243-244 1977.
8. Pensaert, M.B. and DeBouck, P. A new coronavirus-like
particle associated with diarrhea in swine. Arch. Virol. 58: 243-247
1978..
9. Saif L. J. and Wesley, R.D. Transmissible gastroenteritis and
porcine respiratory coronavirus. In: Diseases of Swine, 8th Edition, Iowa State
University Press/ Ames, Iowa USA, pp. 295-325 (Eds: Straw, B. E, D’allairte, S.,
Mengeling. W. L., Taylor, D.J.) 1999.
10. Brenner, J., Elad, D., Van Hamm,
M., Marcovic, A. and Perl, S. Microbiological and pathological findings in
faeces and carcasses of young calves suffering from neonatal diseases. Isr. J.
Vet. Med. 50: 21-24 1995.
11. Liebler-Tenorio, E. M. Pohlenz, J. F. and Whipp
S. C. Diseases of the digestive system. In: Diseases of Swine, 8th Edition, Iowa
State University Press/ Ames, Iowa USA, pp. 821-831 (Eds: Straw, B. E,
D’allairte, S., Mengeling. W. L., Taylor, D.J.) 1999.
12. Kim, L., Chang,
K-OK, Sestak, K., Parwani, A. and Saif, L.J. Development of a reverse
transcription-nested polymerase chain reaction assay for differential diagnosis
of transmissible gastroenteritis virus and porcine respiratory coronavirus from
feces and nasal swabs of infected pigs. J. Vet. Diagn. Invest. 12: 385-388.
2000.
1.
![]() Our Discussion forum is open for
your comments on this article
Our Discussion forum is open for
your comments on this article
